Endocrine function of life-supporting 10-gene-edited porcine kidneys in a nonhuman primate xenotransplantation model
Daniel Eisenson1, Alexander Schulick1, Michelle Santillan1, Kasra Shirini1, Saghar Babadi1, Du Gu1, Kristy Koenig1, William Clarke2, Marc Lorber3, David Ayares3, Kristina DeSmet3, Leigh Peterson3, Hayato Iwase1, Kazuhiko Yamada1.
1Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, United States; 2Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States; 3United Therapeutics Corporation, Silver Spring, MD, United States
Introduction: Xenotransplantation using genetically engineered pigs offers a potential solution to the organ shortage. While survival and immunologic compatibility are critical, the endocrine function of xenografts, specifically their role in erythropoiesis, electrolyte regulation, and the renin-angiotensin-aldosterone system (RAAS), remains poorly characterized. This study evaluated the ability of the 10-gene-edited (10 GE) pig kidney to maintain physiologic endocrine functions in a nonhuman primate (NHP) model of life-supporting kidney xenotransplantation.
Methods: Twelve baboons received life-supporting 10 GE Xenokidneys provided by United Therapeutics Corporation. Endocrine function was evaluated through serial measurements of hemoglobin, reticulocyte counts, serum electrolytes, calcium-parathyroid hormone-vitamin D axis markers, renin, and aldosterone. In vitro assays were used to assess cross-species binding of porcine erythropoietin (EPO) to human EPO receptor. The immunogenicity of porcine EPO and renin was assessed using tiered total and neutralizing antibody assays. Blood pressure was monitored longitudinally. Baboons who received immunosuppression without transplantation were used as controls for comparison.
Results: 10 GE Xenokidneys maintained creatinine clearance and electrolyte homeostasis until onset of xenokidney failure. PTH levels were appropriately suppressed in all recipients, and 25- and 1,25-hydroxyvitamin D levels remained within physiologic range in all but three animals. Porcine EPO bound to the human EPO receptor in vitro. Only one animal required exogenous EPO, and no differences in reticulocyte counts were observed between transplant recipients and controls. Renin was undetectable in all recipients, though aldosterone remained measurable and physiologic blood pressure was maintained throughout the study. No animals developed anti-EPO antibodies. One animal developed transient anti-renin antibodies, which interfered with receptor binding in vitro but did not persist on subsequent testing.
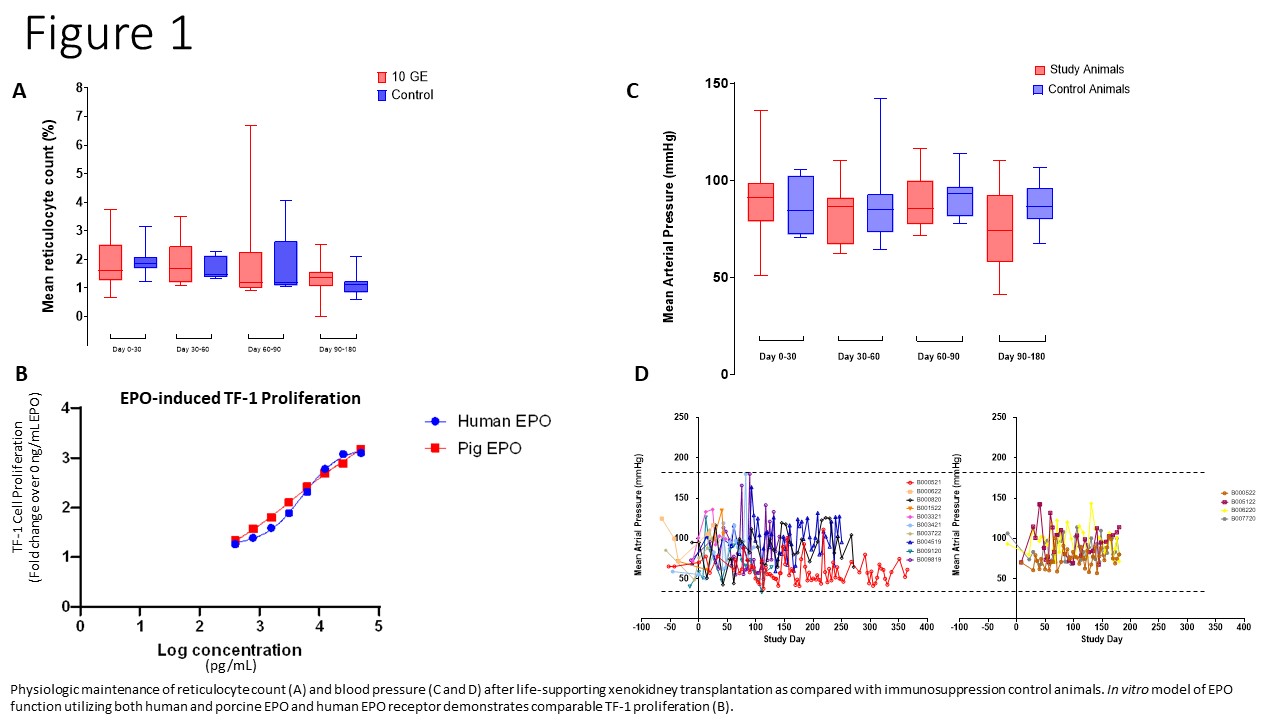
Conclusion: The 10 GE Xenokidney maintained critical endocrine functions in the NHP model, including calcium-PTH-vitamin D axis regulation and support of erythropoiesis. While renin was undetectable, downstream RAAS activity, as evidenced by stable aldosterone levels and normal blood pressure, was preserved. The absence of sustained anti-EPO or anti-renin antibody responses supports the immunologic safety of porcine endocrine protein expression in this context. These findings support the physiologic viability of the 10 GE Xenokidney and inform future clinical application.
This study was supported by a grant from United Therapeutics Corporation.
[1] Endocrine function of porcine kidney
[2] RAAS signaling in porcine kidney
[3] Blood pressure after xenotransplantation
[4] species incompatibilities