Daniel L Eisenson, United States has been granted the IXA Congress Scientific Awards
Incomplete cross-species regulation of complement associated with 10 GE Xenokidney failure in life-supporting pig-to-baboon transplantation
Daniel Eisenson1, WeiLi Chen1, Alexander Schulick1, Michael Cole2, Michelle Santillan1, Kasra Shirini1, Saghar Babadi1, Du Gu1, Kristy Koenig1, Robert Brodsky2, Marc Lorber3, David Ayares3, Kristina DeSmet3, Leigh Peterson3, Andrew Cameron1, Hayato Iwase1, Kazuhiko Yamada1.
1Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, United States; 2Department of Hematology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States; 3United Therapeutics Corporation, Silver Spring, MD, United States
Introduction: Complement-mediated injury is a known barrier to successful xenotransplantation despite advances in source pig genetic engineering and human complement regulatory transgene expression. This study evaluated the contribution of complement activation to xenograft failure following life-supporting kidney transplantation from 10-gene-edited (10 GE) pigs into nonhuman primates (NHPs), within the context of an Investigational New Drug (IND)-enabling nonclinical safety and efficacy study.
Methods: Twelve baboons received a single 10 GE Xenokidney with either calcineurin inhibitor (CNI)-based (Group 1) or CD40/CD40L costimulation blockade-based (Group 2) immunosuppression. All xenokidneys expressed human complement regulators hCD46 and hCD55 but lacked hCD59. Xenokidney biopsies were assessed by immunofluorescence for C3c and C5b-9 deposition. A bioluminescent modified Ham (bmHam) assay was used to model complement activation and test efficacy of pharmacologic inhibitors in baboon serum including C1 esterase inhibitor, anti-C1s, anti-C5, and the alternative pathway inhibitor APL-2.
Results: Complement activation, characterized by early C5b-9 deposition without C3c binding, was observed in the majority of recipients and was associated with microangiopathic injury and xenokidney failure, independent of cellular- or antibody-mediated rejection. Eight of twelve animals demonstrated histologic evidence of thrombotic microangiopathy (TMA) on necropsy without evidence of cellular- or antibody-mediated rejection (no donor-specific antibody formation). Ischemia-reperfusion injury was associated with C5b-9 deposition on early biopsies; local and systemic infections including respiratory viruses were associated with accelerated complement activation and xenokidney failure.
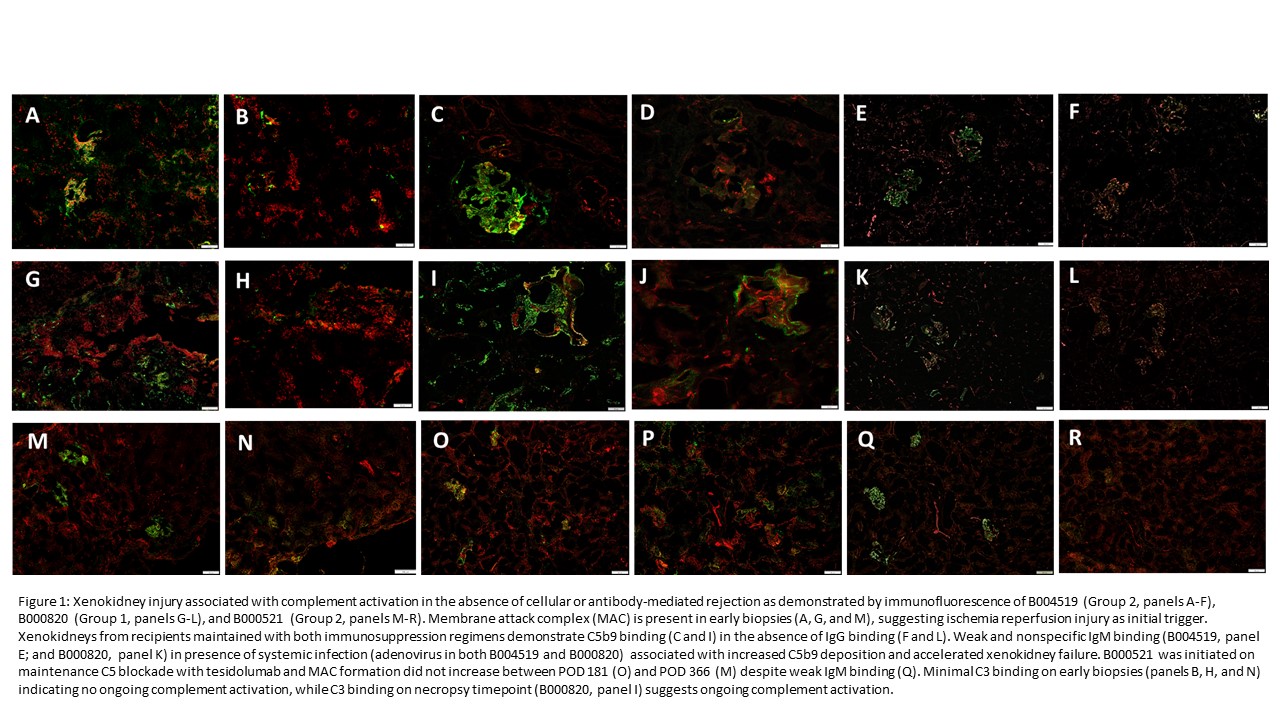
Loss of complement regulatory transgene expression (particularly hCD55) was observed in failing xenokidneys. The bmHam assay demonstrated that standard complement inhibitors (eculizumab, sutimlimab, and APL-2) were ineffective in baboon serum, whereas tesidolumab (anti-C5) provided reliable inhibition.
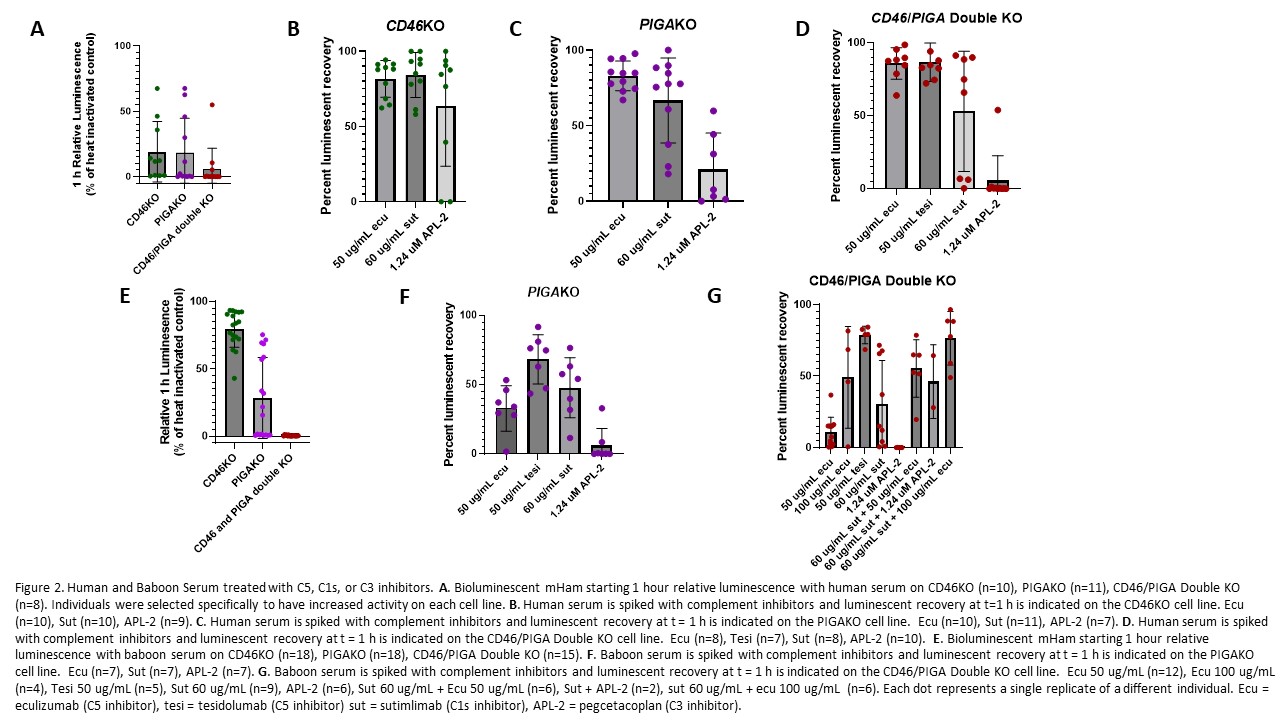
Only one recipient received tesidolumab and maintained long-term xenokidney function with minimal microangiopathy.
Conclusion: Complement activation was the primary driver of 10 GE Xenokidney failure in this pig-to-NHP kidney xenotransplantation study, occurring despite human complement transgene expression and immunosuppression. Inflammation-associated complement activation in the absence of adaptive immune rejection highlights the need for effective and species-compatible complement blockade. These findings support the inclusion of maintenance anti-C5 therapy, and potentially dual-pathway inhibition, in clinical protocols for xenotransplantation.
This study was supported by a grant from United Therapeutics Corporation.
[1] Graft rejection
[2] Xeno-immunosuppression
[3] Complement activation
[4] Pharmacologic complement inhibition