The histologic identification of IgA deposits in three cases of porcine to human Xenokidney transplantation
Aprajita Mattoo1, Vasishta Tatapudi1, Ian Jaffe1, Imad Aljabban1, Tal Eitan1, Jacqueline Kim1, Karen Khalil1, Nicole Ali1, Vineeta Kumar2, Jayme Locke3, Jeffrey Stern1, Adam Griesemer1, Robert A Montgomery1, Edward Skolnik1.
1NYU Langone Transplant Institute , New York, NY, United States; 2Division of Nephrology/Transplant Nephrology, University of Alabama at Birmingham, Birmingham, AL, United States; 3United Therapeutics Corp., Durham, NC, United States
Introduction: Nonhuman primate (NHP) xenotransplants demonstrate the deposition of natural, pre-formed IgA antibodies on biopsies of rejected alpha 1,3-galactosyltransferase gene knockout (GGTA1 KO) pig kidneys. Serum IgA antibodies have been shown to rise in tandem with natural IgG and IgM antibodies during rejection in NHP recipients. Whether these IgA antibodies, like IgG and IgM, play a role in immune-mediated kidney injury remains unknown. Here we describe three cases of pig to human xenokidney transplants and the pattern of IgA deposition on biopsy during their clinical care.
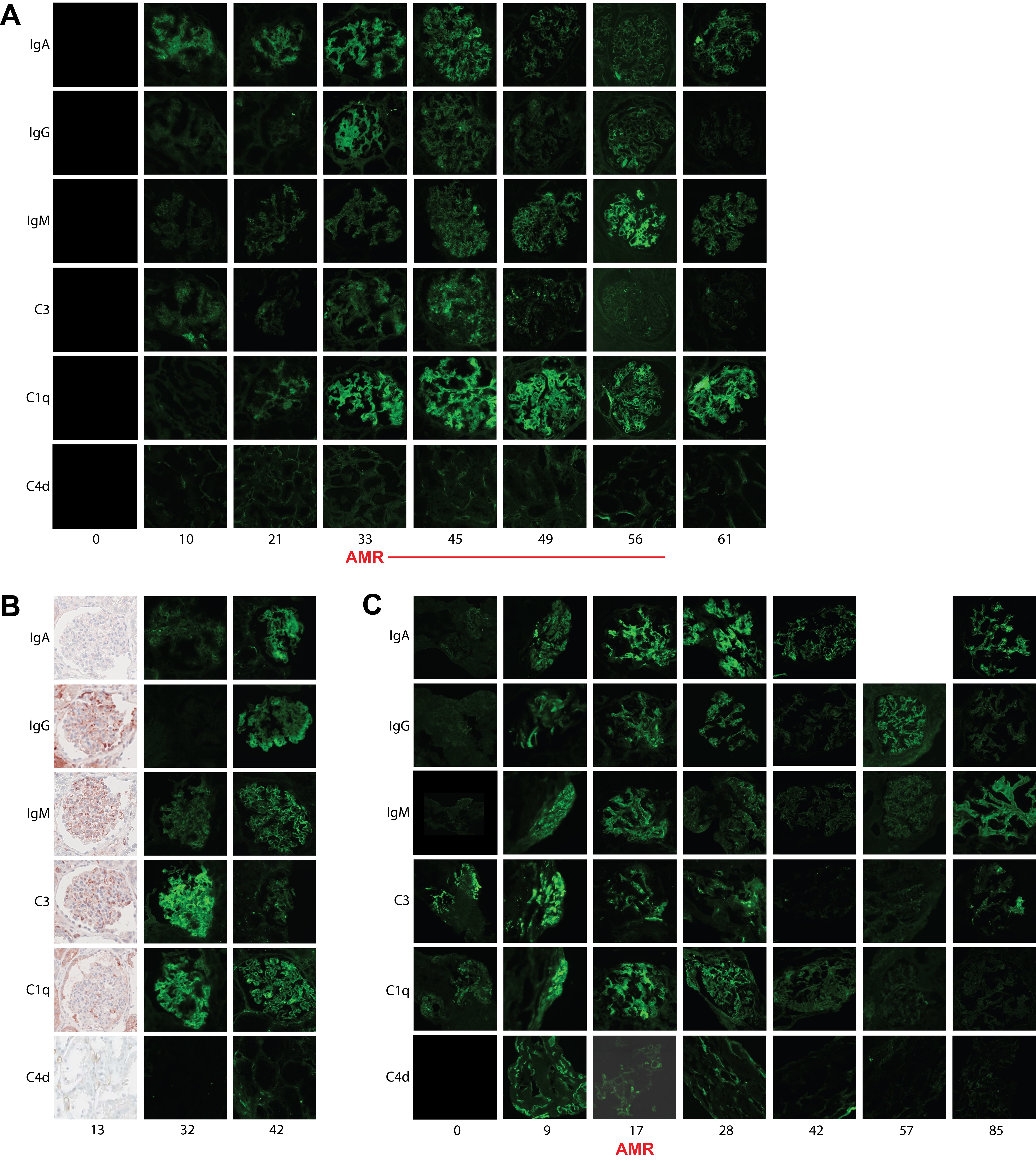
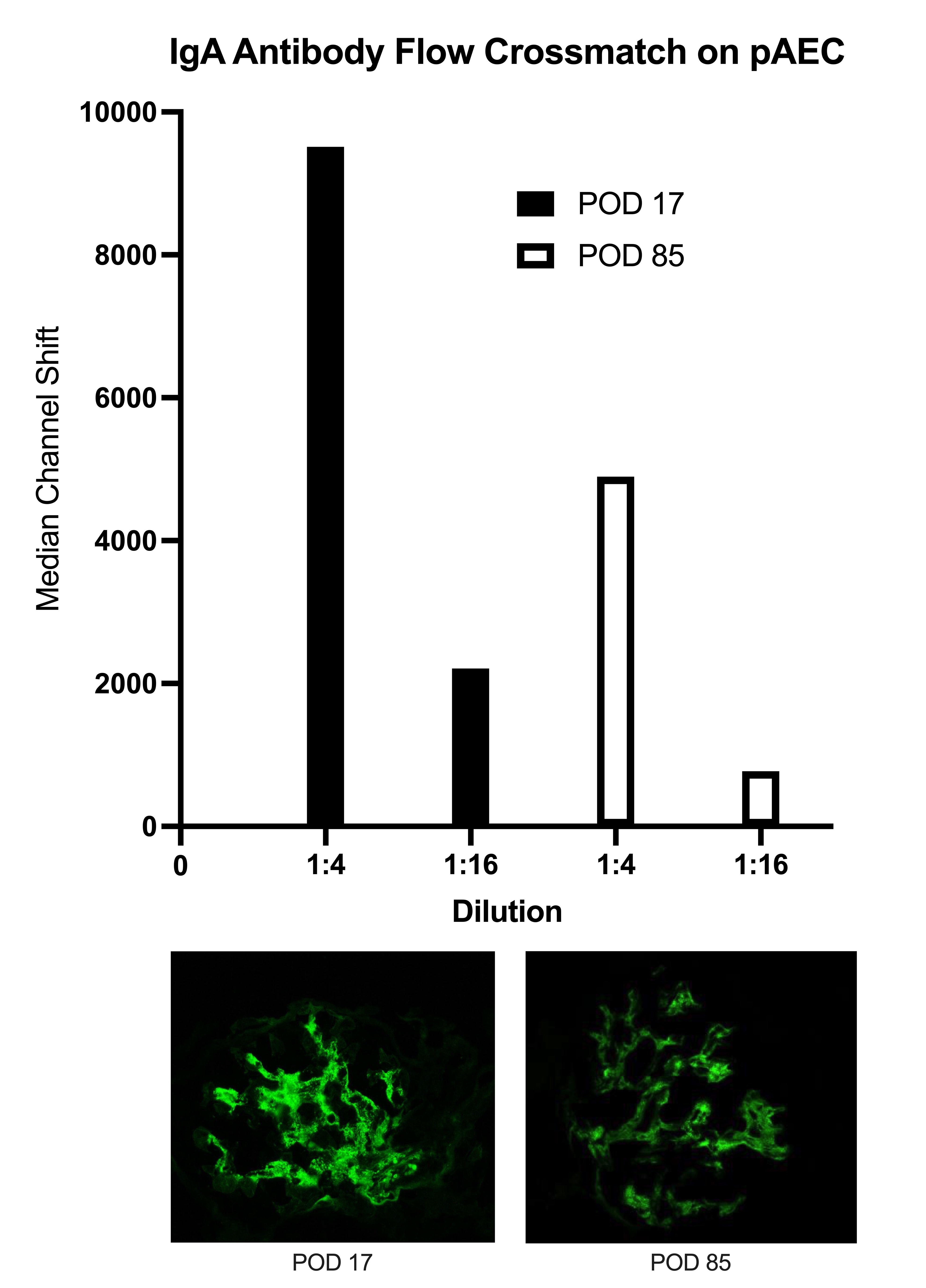
Methods: Three humans underwent xenokidney transplants with two different genetically modified pig kidneys. Case A, a decedent with a GGTA1 KO Thymokidney maintained in the ICU for 61 days, was successfully treated for antibody-mediated rejection (AMR) on POD33 and subsequently maintained excellent graft function until planned explant on POD61. Case B, a living recipient with a GGTA1 KO Thymokidney after LVAD placement, underwent explant on POD47 due to progressive graft failure in the setting of hemodynamic instability and was later identified to have an antibody-mediated process on molecular transcriptomics. Case C, a living recipient with a 10 GE Xenokidney, was successfully treated for AMR on POD17 and subsequently maintained excellent graft function until development of a second severe rejection on POD128 and underwent explant on POD130. All three cases underwent protocol and for-cause biopsies with immunoglobulin and complement staining. In Case C, serum IgA titers were correlated with IgA staining on biopsy at the time of AMR diagnosis and after treatment.
Results: All biopsies demonstrated evidence of glomerular mesangial proliferation. Case A became IgA positive starting on POD10. IgA diminished in positivity after treatment of AMR. This deposition was similar to IgG, IgM, C3 and C4d staining. Case B was negative for IgA on POD13 but developed IgA positivity by POD32 along with IgM and C3 positivity. IgA positivity increased by POD42, as did IgG. Case C became IgA positive starting on POD9. IgA diminished in positivity after AMR treatment. This deposition was similar to IgG, IgM, C3 and C4d staining (Figure 1). Serum IgA titers in Case C on POD17 (AMR onset) and POD85 (AMR resolution) were found to correlate with IgA positivity on biopsy (Figure 2).
Conclusion: The relevance of IgA antibodies in immune-mediated kidney injury in pig to human xenotransplants is unknown. In two of three pig to human xenotransplant cases at our institution, IgA staining preceded AMR diagnosis and diminished after treatment, following a pattern similar to other immunoglobulin and complement staining consistent with AMR diagnosis. Serum IgA titers also appeared to diminish after AMR treatment and correlated with decreased IgA staining on biopsy.
This data is supported by funding from United Therapeutics Corporation, PBC. Tal Eitan was supported by the National Cancer Institute, National Institutes of Health, through Grant Award Number T32 CA193111.
[1] IgA, Xenokidney