Incidence and management of proteinuria in a living recipient of a 10 gene-edit xenokidney transplant
Vasishta Tatapudi1, Karen Khalil1, Imad Aljabban1, Tal Eitan1, Ian Jaffe1, Vineeta Kumar2, Nicole Ali1, Jayme Locke3, Jeffrey Stern1, Adam Griesemer1, Robert A Montgomery1, Edward Skolnik1, Aprajita Mattoo1.
1NYU Langone Transplant Institute, New York, NY, United States; 2Division of Nephrology/Transplant Nephrology, University of Alabama at Birmingham, Birmingham, AL, United States; 3United Therapeutics Corp., Durham, NC, United States
Introduction: The etiology of high-grade proteinuria reported in nonhuman primate xenotransplantation is poorly understood. Significant proteinuria was not reported in the first two renal xenotransplants recipients performed at MGH and NYULH. Here, we report the incidence and management of proteinuria in the living recipient of the longest-surviving renal xenotransplant.
Methods: A 53-year-old Black woman donated a kidney in 1999. In 2004, she developed pregnancy-induced hypertension, eventually leading to hemodialysis-dependent end-stage kidney disease in 2016. The presumed cause of her kidney disease was APOL1-mediated, as genomic analysis revealed two risk alleles. On November 25, 2024, she underwent a 10 gene-edit xenokidney transplant. We monitored urine protein and albumin excretion post-transplant and performed protocol and for-cause biopsies.
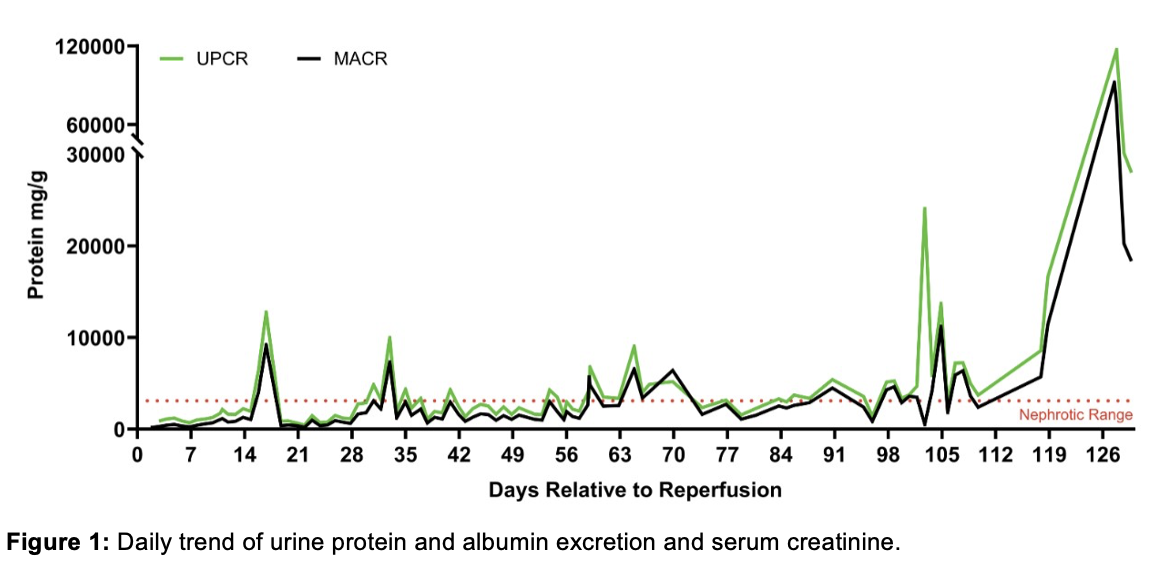
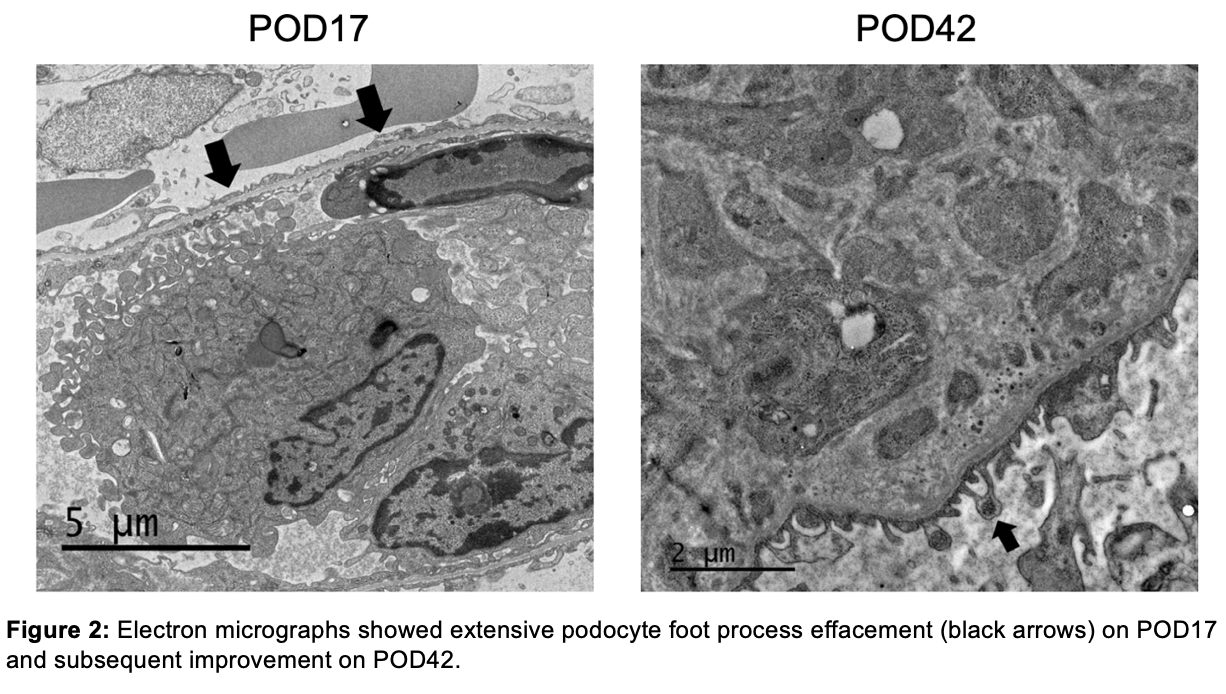
Results: The xenograft functioned immediately. Serum creatinine (SCr) decreased from 7.6 mg/dL to 1.0 mg/dL within 72 hours. Electron microscopic (EM) examination of a protocol biopsy on POD 9 demonstrated intact podocyte foot processes with no evidence of glomerular basement membrane (GBM) remodeling or electron-dense immune deposits. Within the first two weeks, the mean urine protein/creatinine (u p/cr) was 1.3 ± 0.44 g/g. On POD 17, although SCr remained stable at 1.0 g/dL, the u p/cr acutely increased to 12.7 g/g (Fig.1), prompting a biopsy. EM revealed near total foot process effacement (Fig.2). Since a circulating factor or podocyte-specific xeno-antibody-associated focal and segmental glomerulosclerosis could not be ruled out, five sessions of therapeutic plasma exchange (PLEX) were initiated. This resulted in a decrease in u p/cr to 0.9 g/g within 72 hours. The u p/cr averaged 0.98 g/g until POD 33, when it acutely increased to 9.9 g/g. PLEX again improved u p/cr to 1.1 g/g. After this, the patient had variable but significant proteinuria (5.8 ± 5.8 g/g), though her graft function remained excellent with a stable SCr of 1.59 ± 0.32 mg/dL. However, on POD 127, she presented with an acute rise in SCr to 10.3 mg/dL in the setting of decreased immunosuppression due to infection. She was diagnosed with acute rejection resulting in xenograft failure. The increase in u p/cr in the final days noted in Fig.1 likely represents a significant overestimate since the urine samples were obtained during oliguric acute kidney injury.
Conclusion: We report episodic nephrotic range proteinuria in the longest-functioning pig-to-human kidney transplant. Proteinuria was initially responsive to PLEX and did not impede graft function. Further studies are needed to elucidate the etiology of proteinuria, which may include antibody-mediated rejection, immune complex-mediated glomerulonephritis, or podocyte-specific xeno-antibodies. Determining the mechanism of injury to the filtration barrier holds the key to developing targeted therapies aimed at preventing or reversing proteinuria should it occur.
This data is supported by funding from United Therapeutics Corporation, PBC. Tal Eitan was supported by the National Cancer Institute, National Institutes of Health, through Grant Award Number T32 CA193111.
[1] Xenokidney, proteinuria