Xenoperfusion of triple glycan knock-out, seven human transgene insertion porcine livers with human whole blood
Alex Sagar1, John Fallon1, Mohamed Elzawahry1, Syed Hussain Abbas1, Leanne Lanieri2, Kathryn Stiede2, Kristen Getchell2, Paula Hamilton2, Carson Sutter2, Amanda Marett2, Michael Etter2, Cassandra Miller2, Kayla Janke2, Rafferty Wyatt2, Raquel Castro2, Chris Morris3, Dan Fower3, Shawn Welch3, Lydia Lamriben2, Eric Brass7, Gregory Hendricks6, Kelly Cederquist4, Frederick Vyas4, Peter Abt4, Charles Abrams5, Abraham Shaked4, Susan Low2, Peter Friend1,3.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2eGenesis, Cambridge, MA, United States; 3OrganOx, Oxford, United Kingdom; 4Penn Transplant Institute, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 5Hematology-Oncology Division, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 6Department of Cell Biology, University of Massachusetts Medical School, Worcester, MA, United States; 7Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Introduction: Short-term xenografting of transgenic porcine livers holds promise as a therapy to temporarily support patients with acute and acute-on-chronic liver failure until recovery of native liver function or allotransplantation. Ex situ xenoperfusion is a technique in which a xenograft is perfused with human whole blood under physiological conditions outside the body. This may provide a platform, in parallel to in vivo experiments, to investigate xenograft viability, erythrophagocytosis, thrombocytopenia and drug metabolism.
Methods: Porcine livers (EGEN-5784) were procured from Yucatan minipig clones with the following genetic edits: (i) triple glycan knockout; (ii) hemizygous insertion of seven human transgenes; (iii) functional inactivation of porcine endogenous retrovirus pol gene (3KO.7TG.RI). The EGEN-5784 livers underwent normothermic perfusion with pooled human whole blood on a metra (OrganOx, Oxford, UK).
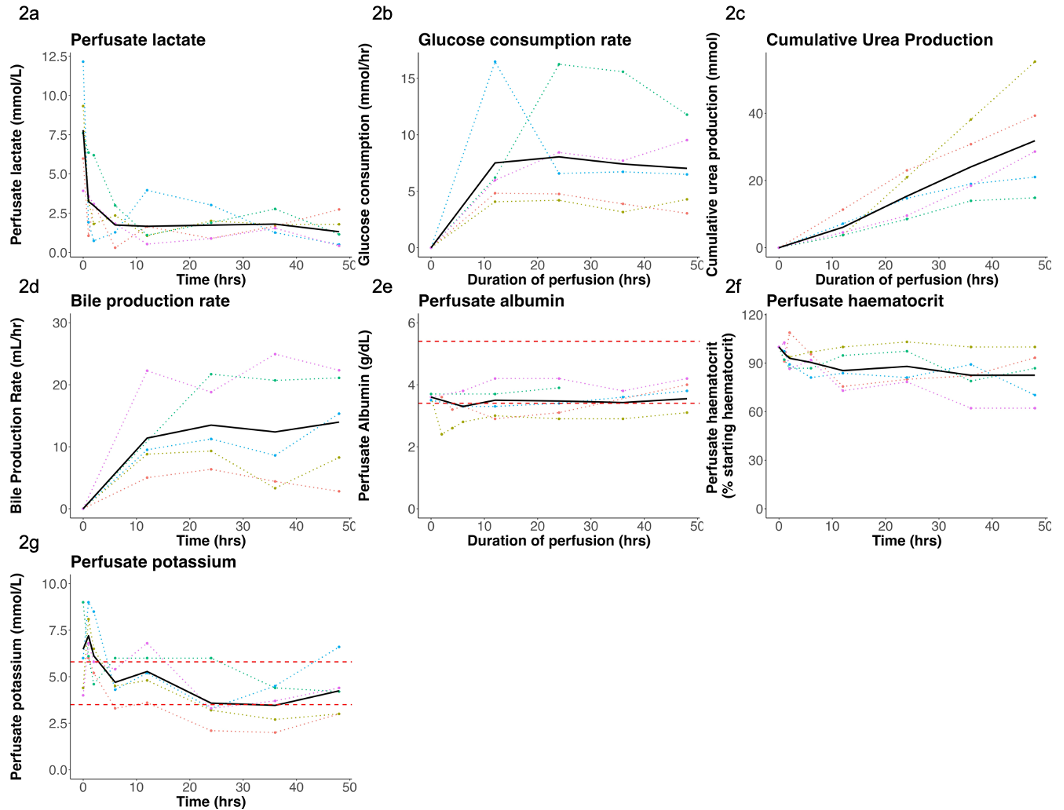
Five livers (3KO.7TG.RI: n = 4; 3KO.7TG: n = 1) underwent xenoperfusion for 48 hours to assess xenograft viability and erythrophagocytosis. During xenoperfusions 4 and 5, CYP3A4 and CYP2C19 activity was assessed by measuring midazolam and omeprazole clearance.
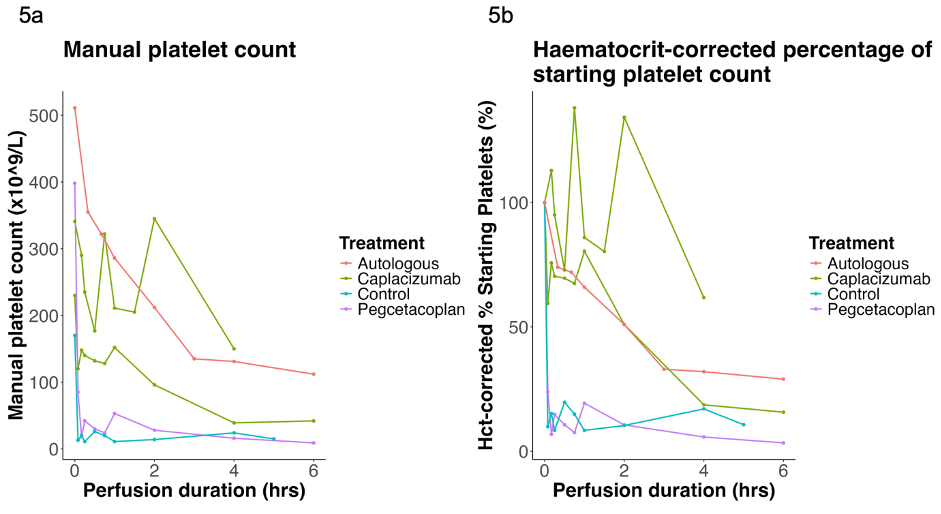
To assess the mechanism of early xenograft-associated thrombocytopenia, a further four livers (3KO.7TG.RI: n = 4) underwent 4-6-hour xenoperfusion under three different conditions: (i) control: standard normothermic liver perfusion (n=1), (ii) capalacizumab pre-treatment (von Willebrand factor inhibitor) (n=2), and (iii) pegcetacoplan pre-treatment (complement C3 inhibitor) (n=1). A single whole blood autologous wild-type pig liver perfusion was used as an ‘autoperfusion’ control.
Results: Viable xenoperfusion was maintained for 48 hours for all five livers. The livers maintained physiological hepatic artery (0.27± 0.11 L/minute) and portal vein (0.97± 0.04 L/minute) flows, and demonstrated hepatic function throughout perfusion. The 48-hour perfusate haematocrit was 83 ± 16 % of the starting value.
Intrinsic xenograft clearance of midazolam was 16.14 ± 2.83 L/min/kg and 10.85 ± 2.24 L/min/kg for xenoperfusions 4 and 5, respectively. Intrinsic xenograft clearance of omeprazole was 2.36 ± 0.87 L/min/kg and 1.89 ± 0.76 L/min/kg for xenoperfusions 4 and 5, respectively.
Control and pegcetacoplan pre-treated xenoperfusions demonstrated a rapid, profound decline in perfusate platelet count. Capalacizumab pre-treatment delayed the onset of thrombocytopenia. In these xenoperfusions, progressive platelet aggregation occurred in the hepatic sinusoids within the first hour, associated with sinusoidal endothelial von Willebrand factor staining.
Conclusions: Viable xenoperfusion of EGEN-5784 porcine livers can be maintained for 48-hours, with evidence of preserved hepatic function and CYP450 activity. Early xenograft-associated thrombocytopenia appears to be mitigated ex situ by von Willebrand factor inhibition; this warrants further investigation in vivo.