Extra-corporeal liver cross-circulation using transgenic porcine xenografts in the human decedent model
Alex Sagar1, Abraham Shaked2, Kim Olthoff2, Peter Abt2, Emily Vail3, Niels Martin4, Charles Abrams5, Rick Hasz6, Mohamed Elzawahry1, John Fallon1, Syed Hussain Abbas1, Christine Radolovic6, Shannon Kaminski6, James Markmann2, Susan Low7, Leanne Lanieri7, Kathryn Stiede7, Kirsten Swenson7, Danielle Fortuna8, Rajender Reddy2, Peter Reese2,9, Peter Friend1,10.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2Penn Transplant Institute, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 3Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 4Department of Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 5Hematology-Oncology Division, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 6Gift of Life Donor Program, Philadelphia, PA, United States; 7eGenesis, Cambridge, MA, United States; 8Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States; 9Vanderbilt Center for Transplant Science, Vanderbilt University Medical Center, Nashville, TN, United States; 10OrganOx, Oxford, United Kingdom
Introduction: There is an unmet need for effective temporary liver support to bridge patients with acute and acute-on-chronic liver failure to native liver recovery or allotransplantation. In extra-corporeal liver cross-circulation (ELC) the patient’s blood is perfused through a donor liver maintained by physiological perfusion outside the body. Transgenic porcine livers may be a viable source of livers suitable for ELC.
Methods: Four brain-dead research donors (‘decedents’) underwent ELC using porcine livers (EGEN-5784) with the following genetic modifications: (i) triple glycan knockout; (ii) hemizygous insertion of seven human transgenes; (iii) functional inactivation of porcine endogenous retrovirus pol gene. Immunosuppression comprised intravenous methylprednisolone.
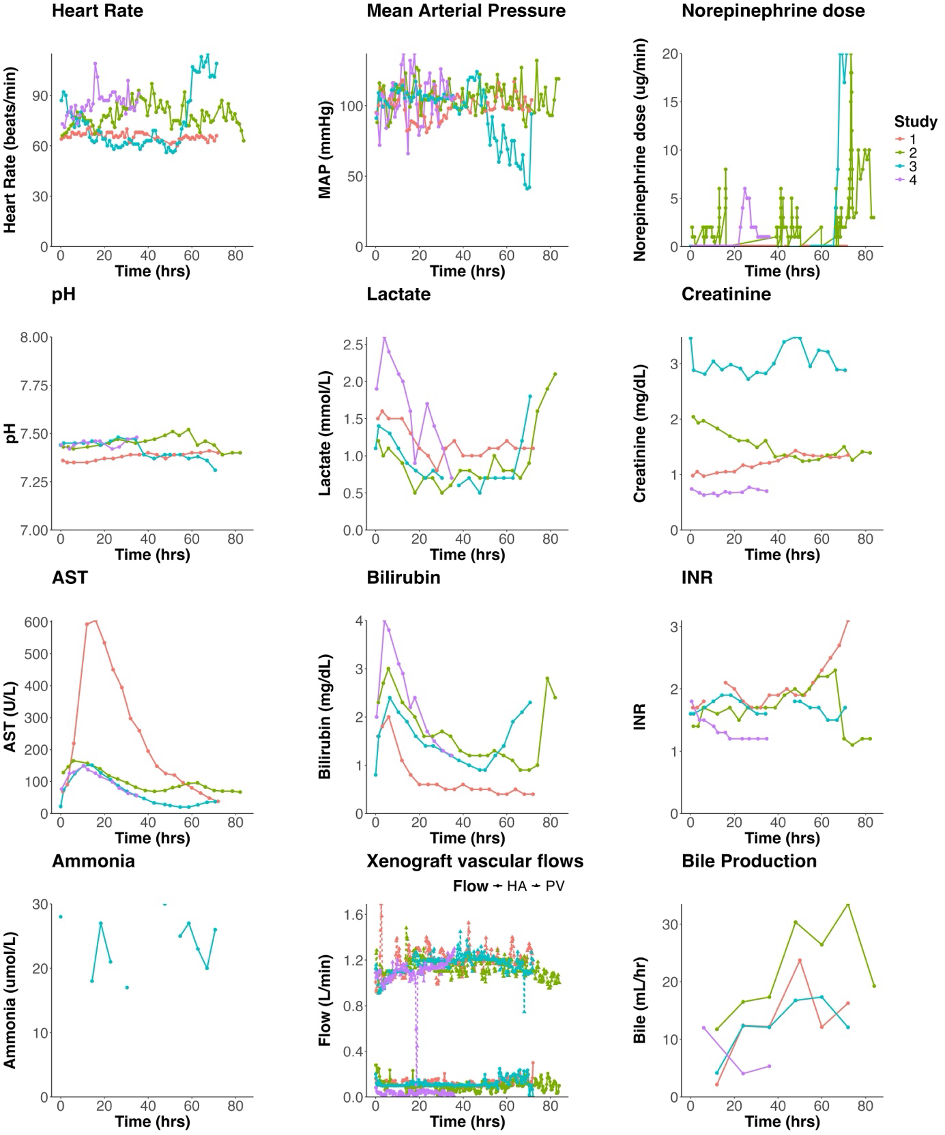
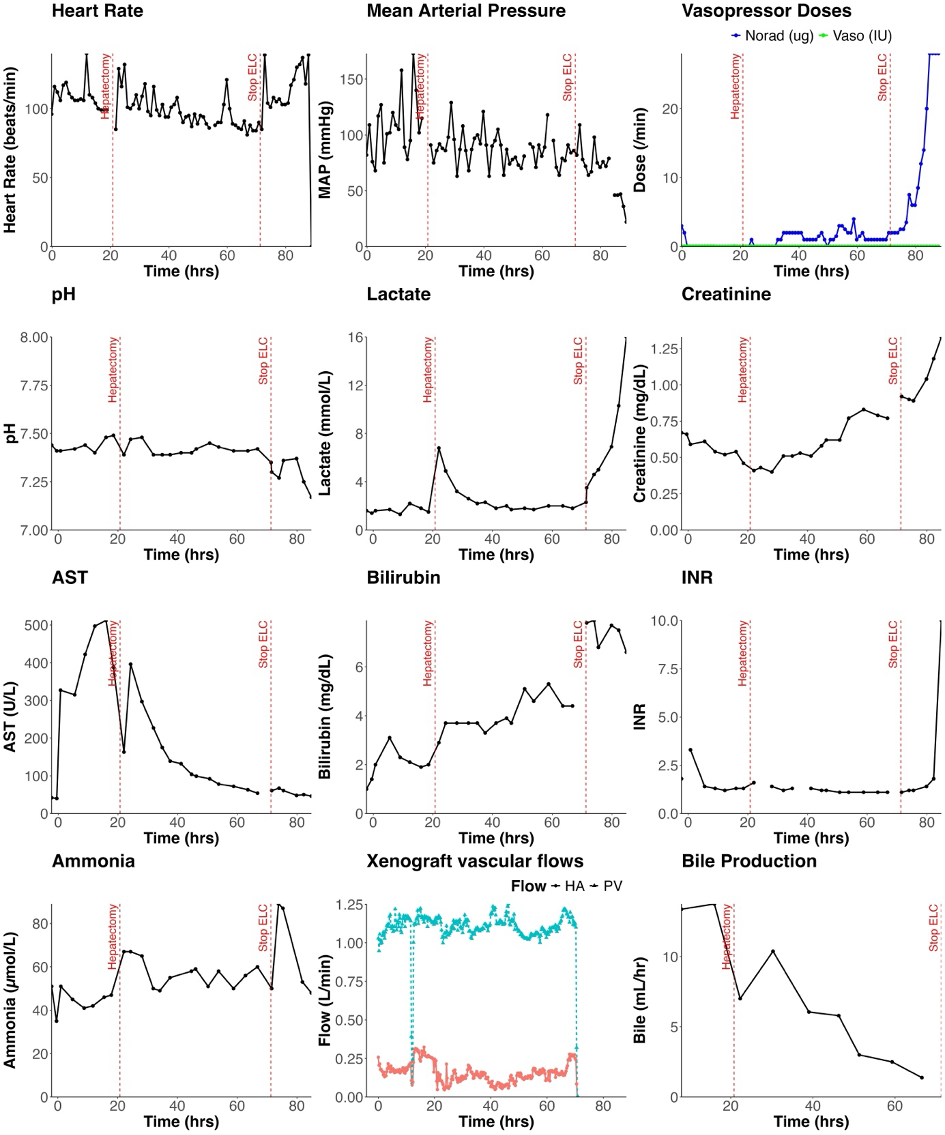
Decedents 1 – 3 underwent ELC with their native livers intact for 72-84 hours (‘ELC 1 – 3’). Decedent 4 underwent two consecutive sessions of ELC with two EGEN-5784 porcine livers for a total ELC duration of 100 hours (‘ELC 4 and 5’). 20.8 hours into the second ELC session, decedent 4 underwent native hepatectomy with porto-caval anastomosis followed by a further 48 hours of ELC. After discontinuation of ELC, decedent 4 was observed with continued critical care support until cessation of circulation.
Results: During ELC in decedents with intact native livers, the EGEN-5784 porcine livers maintained bile production and augmented native hepatocellular cytosolic, mitochondrial, microsomal metabolism and cholate clearance.
Post-native hepatectomy, ELC maintained Decedent 4 with minimal inotrope requirement, and normal lactate, pH, base excess, INR and ammonia: this persisted until elective termination of ELC 48 hours post-hepatectomy.
Thrombocytopenia developed within one hour of commencing ELC in all decedents. The mean platelet count during ELC was 24,000/μL (range of study means: 10-33,000/μL) with only transient response to platelet transfusion. EGEN-5784 porcine liver biopsies demonstrated largely preserved hepatic parenchyma with mild inflammatory infiltration of predominantly CD3-and CD14-postive cells and evidence of IgM deposition.
Conclusions: ELC is feasible for at least 72 hours using transgenic porcine livers in a human context and can provide effective temporary liver support for at least 48 hours. This approach may provide an effective bridging therapy in acute and acute-on-chronic liver failure.
The authors acknowledge the contributions of Emily Blumberg, MD; Greg Everson, MD; Lexi Tumblety; David Wagner PhD; Mike Duggan DVM, and the staff at Accuro Farms; Chris Morris; Dan Fower; Shawn Welch; Leah Lambe, Jamie Ann Acero-Webb, and the staff at the Gift of Life Donor Care Center.