
Preliminary results of obtaining designated pathogen-free pigs in a pig facility for xenotransplantation in Brazil
Michelle S. Araujo1, Luiz Gustavo C. Munhoz1, Ligiane O. Leme1, Vitor Leão1, Gabriel H. Santos1, Mayumi S. Hayami1, Valéria V. Chida1, Geissiane M. Marcondes2, Marina J. Baumhak1, Ludmila V. Garcia1, Karina A. O. Braga1, Luciano Brito1, Mayana Zatz1, Silvano M. A. Raia1, Ernesto Goulart1.
1Genetics and evolutionary biology, Institute of biosciences, University of São Paulo, Sao Paulo, Brazil; 2Veterinary medicine, Unimax and Unifaj (Unieduk group), Sao Paulo, Brazil
Introduction: In 2024, the first Brazilian pig facility (PF) was inaugurated with the aim of producing genetically modified pigs for xenotransplantation. This study aims to report the preliminary results of the first standardization procedure to produce DPF pigs at the Xeno Brazil PF, located in São Paulo-SP, Brazil.
Methodology: Embryos produced by in vitro fertilization were transferred to previously synchronized non-SPF recipient sows via laparotomy. The piglets were delivered by hysterectomy in the surgical center in the clean area of the PF. After removal, the uterus was immersed in a waterlock bath containing an antiseptic solution (Virkon at 10%) and opened in an adjacent neonatology room for the birth of three piglets. Artificial lactation was carried out without colostrum (Denkapig - 450g/L for the first 5 days and 250g/L until 30 days). To determine the DPF status of the piglets and the sow, samples of whole blood, serum, feces, nasal and oral swabs, and tissue (lung, diaphragm, and heart) were collected. The piglets and sow samples were pooled and tested for 71 pathogens using a molecular panel (qPCR) based on studies in the literature on xenotransplantation. Surface swab samples were collected from the operating room, waterlock, neonatology room, piglet incubators, milk preparation room, materials storage, and dressing areas after sanitization (neutral detergent, 0.2% sodium hypochlorite, and 1% virkon) and before stocking the animals, and tested for 4 environmental pathogens. The choice of pathogens tested was based on the scientific literature, as well as considering the reality of Brazilian pig health, since Brazil does not yet have its specific legislation for xenotransplantation.
Results: The sow was positive for 7 pathogens (Campylobacter spp., Mycoplasma spp., Mycoplasma hyorhinis, Pasteurella multocida, Salmonella enterica, Streptococcus spp., and PCV2). The piglets were negative for all the evaluated pathogens (Table 1). None of the pathogens tested in the PF environment were detected (Table 2).
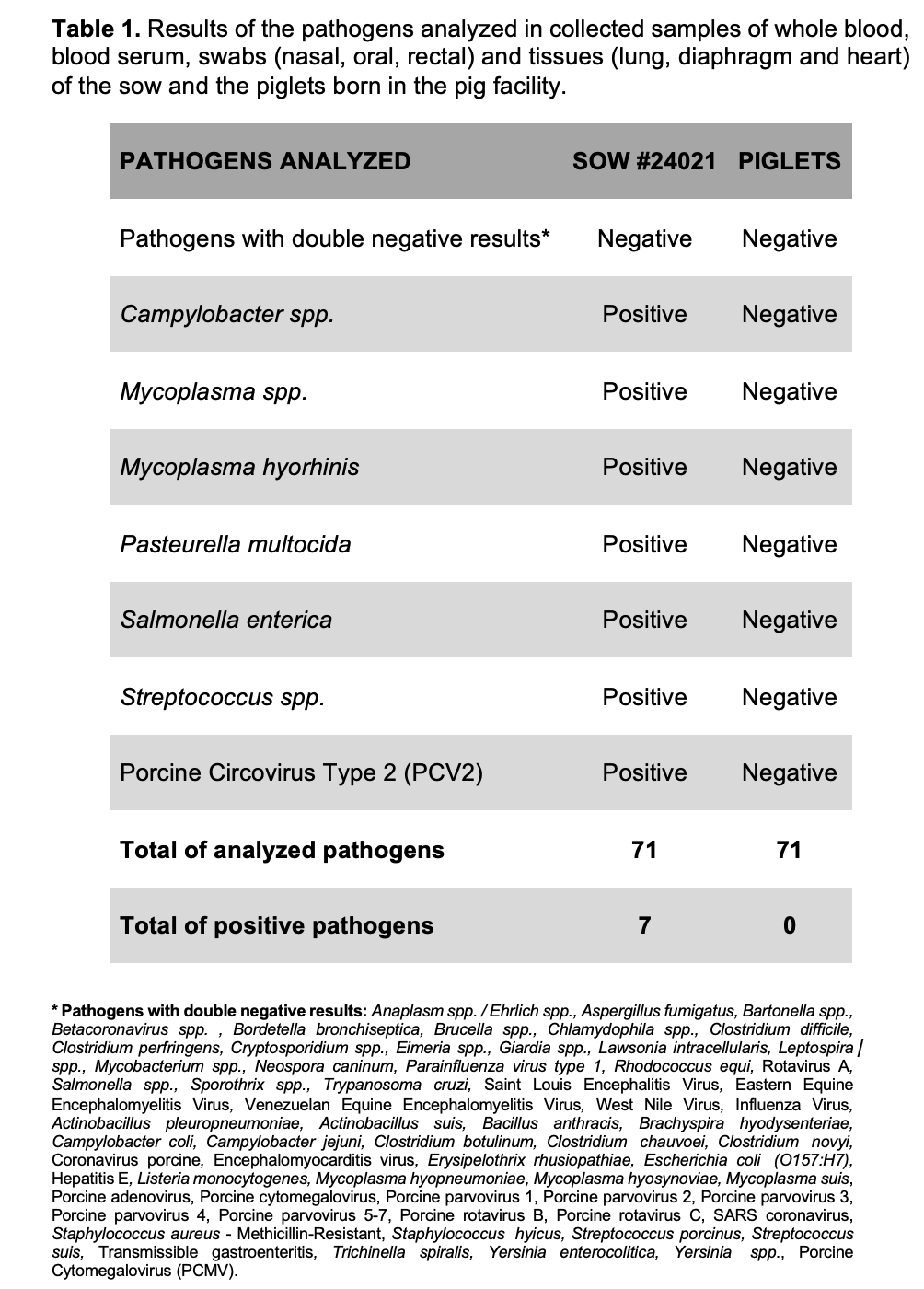
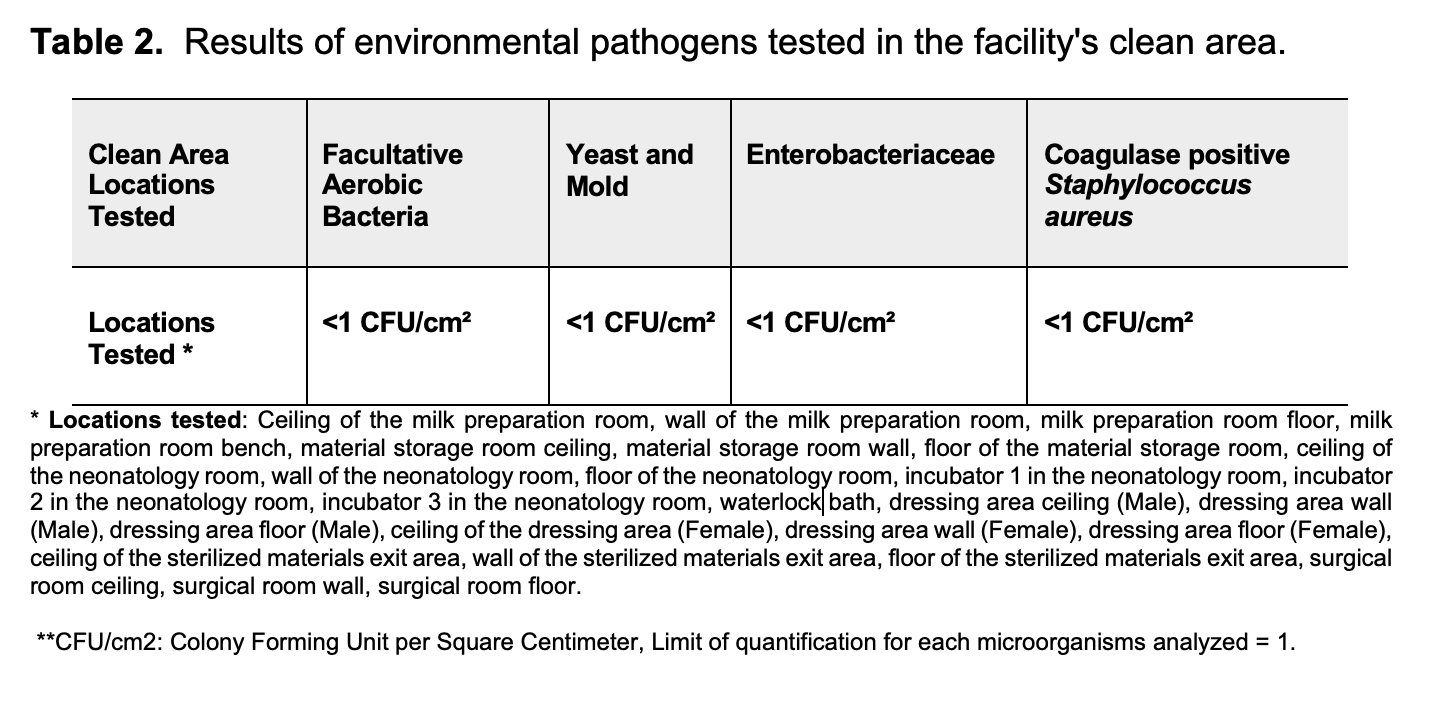
Conclusion: The method to obtain DPF piglets and the sanitization of the PF have proved to be efficient so far. Although the sow tested positive for 7 pathogens, none of the 3 piglets born were positive. New piglet samples will be taken at 2 months of age to confirm their DPF status. Prior standardization of the techniques for obtaining DPF pigs facilitates the training of facility staff, as well as optimizing techniques and the use of resources, reducing costs, and minimizing errors when obtaining genetically modified pig clones for xenotransplantation.
São Paulo Research Foundation (FAPESP) - grant number 21/11872-5. National Council for Scientific and Technological Development (CNPq) - grant number 444075/2023-2. Água Branca Pig Farm, Itu, São Paulo, Brazil. Biotec Group, Manaus, Brazil.
[1] Swine
[2] Xenozoonoses
[3] Pig facility