
Standardization of multiplex qPCR assays for detection of PCMV and PCV2 in organ donor pigs
Mayumi S. Hayami1, Luiz Gustavo C. Munhoz1, Gabriel H. Santos1, Michele S. Araújo1, Ludmilla V. Garcia1, Vitor Leão1, Ligiane O. Leme1, Karina A. O. Braga1, Luciano Brito1, Mayana Zatz1, Silvano M. A. Raia1, Ernesto Goulart1.
1Genetics and evolutionary biology, Institute of Biosciences - University of São Paulo, São Paulo, Brazil
Introduction: Ensuring non-contamination by xenozoonotic pathogens in organs intended for xenotransplantation is crucial. Real-time PCR (qPCR) remains the most widely used method for active infections diagnosis, offering high sensitivity and specificity. Given the wide variety of pathogens to be tested, several types of material are required to ensure reliable results. Consequently, the development of an "in-house" multiplex molecular panel capable of detecting pathogens deemed high-risk for xenotransplantation emerges as a cost-effective and expeditious alternative to the conventional testing methods involving animals and pig facilities. This study presents the progress made in optimizing a multiplex qPCR protocol for the simultaneous detection of Porcine Cytomegalovirus (PCMV/PRV), Porcine Circovirus type 2 (PCV2) and the endogenous gene pGAPDH evaluating different DNA extraction kits, primer sets, and biological sample types to identify the most reliable combination.
Methods: To compare the extraction efficiency, blood, serum, semen, and tissue samples collected from 10 animals were subjected to DNA extraction using five different commercial kits and assessed nucleic acid yield, purity, and the sensitivity by the endogenous gene pGAPDH amplification. The qPCR limit detection and assay efficiency were evaluated between two primers sets for PCMV (Mueller, 2002; Morozov, 2016) and PCV2 (Olvera, 2004; Li, 2018) using gBLOCK with 10-fold serial dilution in triplicate, additionally with widely used commercial master mixes. Thus, the most reliable extraction kit, primer set and mastermix combination were used to extract and amplify 37 samples of blood, serum and tissues in singleplex and multiplex, to measure possible sensitivity loss. Samples with CT values near 38-39 were retested multiple times to ensure reproducibility.
Results: The GeneJET viral extraction kit associated with Mueller (2002) and Li (2018) primers and AgPath-ID mastermix showed most reliability and detection of less copy numbers. No sensitivity loss was found when comparing multiplex assays. Among the 37 samples evaluated, 5 (13,5%) and 12 (32,4%) were considered positive, with a reproducibility rate for low number copies samples of 78,9% and 100% to PCMV and PCV2 respectively.
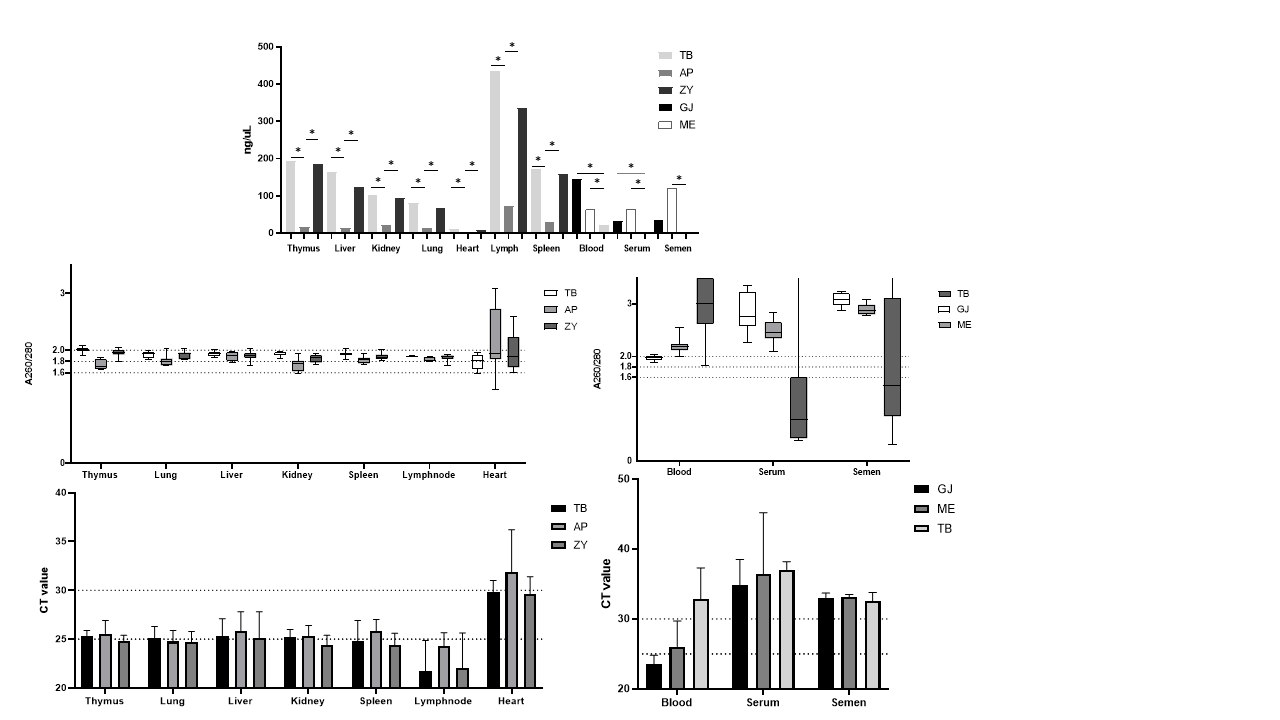
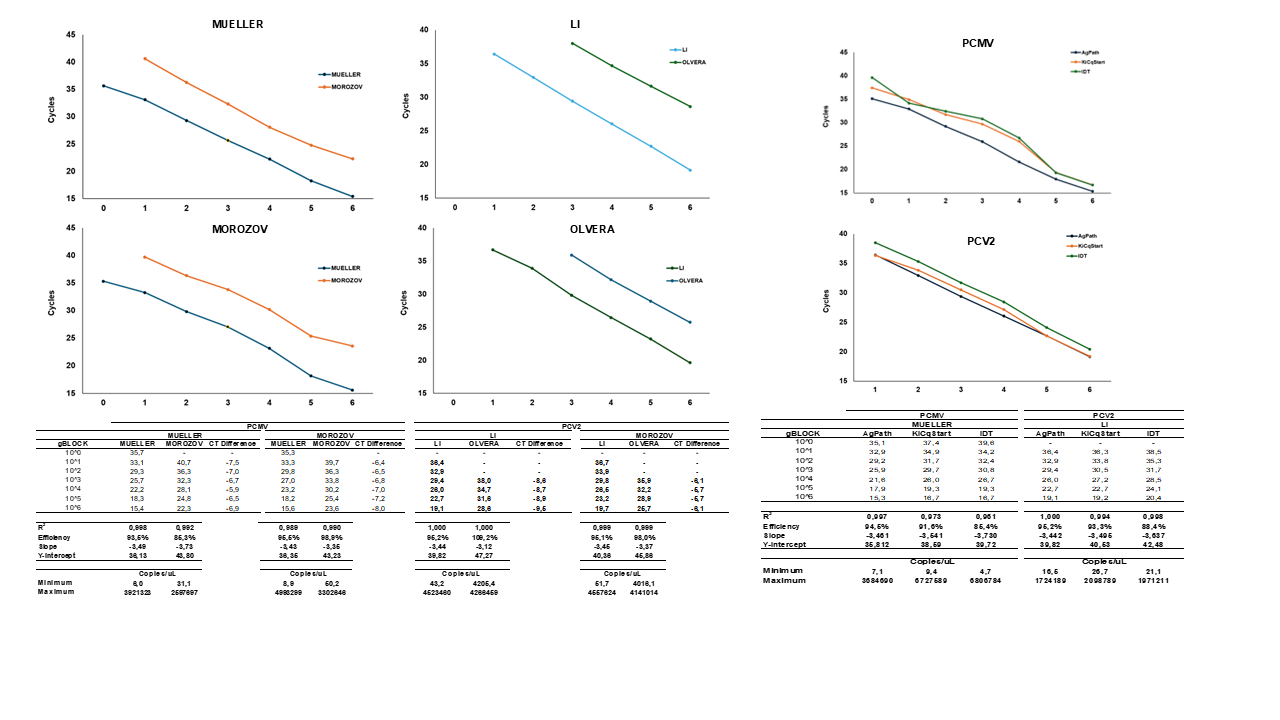
Conclusion: To date, our study has successfully standardized multiplex qPCR for detecting PCMV and PCV2 with limits of 100 and 101 copies respectively, with no sensitivity loss, even in materials difficult to amplify. Our next step is monitoring the presence of these pathogens in nursery animals and expanding the standardization to 11 pathogens in panel format.
São Paulo Research Foundation (FAPESP) - grant number 21/11872-5. National Council for Scientific and Technological Development (CNPq) - grant number 444075/2023-2. Água Branca Pig Farm, Itu, São Paulo, Brazil.
[1] PCMV
[2] PCV2
[3] qPCR
[4] Xenotransplant
[5] Diagnosis
[6] DPF