Dosing of pegcetacoplan with plasmapheresis for treatment of xenoantibody mediated rejection
Karen Khalil1, Rebecca Dieter1, Peter Hillmen2, Pascal Deschatelets2, Imad Aljabban1, Tal Eitan1, Ian Jaffe1, Elaina P Weldon1, Vineeta Kumar3, Jayme Locke4, Nicole Ali1, David Ayares4, Sapna A Mehta1, Brendan Keating1, Massimo Mangiola1, Alexandre Loupy5, Ming Wu6, Jeffrey M Stern1, Adam Griesemer1, Aprajita Mattoo1, Vasishta Tatapudi1, Robert A Montgomery1, Edward Skolnik1.
1NYU Langone Transplant Institute, New York, NY, United States; 2Apellis Pharmaceuticals, Waltham, MA, United States; 3Division of Nephrology/Transplant Nephrology, University of Alabama at Birmingham, Birmingham, AL, United States; 4United Therapeutics Corporation, Research Triangle Park, NC, United States; 5Paris Institute for Transplantation and Organ Regeneration, Paris, France; 6Anatomic Pathology, Northwell Health, New Hyde Park, NY, United States
Introduction: Antibody mediated rejection (AMR) is a concern in the implementation of clinical xenotransplantion. Plasmapheresis (PP) is an integral part of AMR management, however, limited data exists on the effect of PP on many novel complement inhibitors including pegcetacoplan (PEG), an inhibitor of complement components C3 and C3b.
Methods: An expanded access single patient investigational new drug (IND) application was approved for a 10 gene-edit (10 GE) porcine kidney transplanted into a 53-year-old ESRD patient, previous kidney donor on dialysis, with a cPRA of 99.98%. A clinically approved immunosuppression regimen consisting of lymphocyte depletion, corticosteroids, belatacept, tacrolimus, mycophenolate and a C5 inhibitor was utilized.
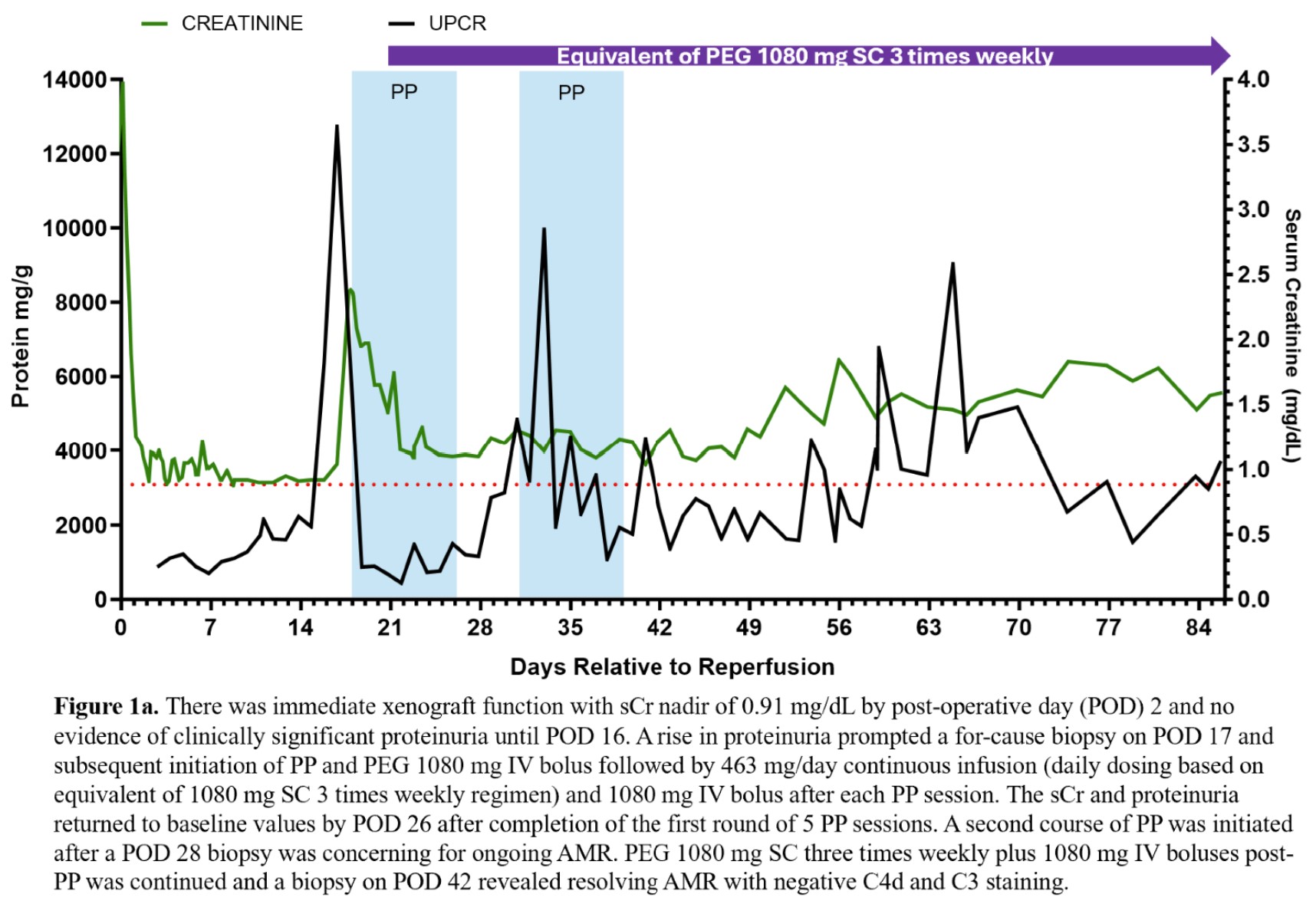
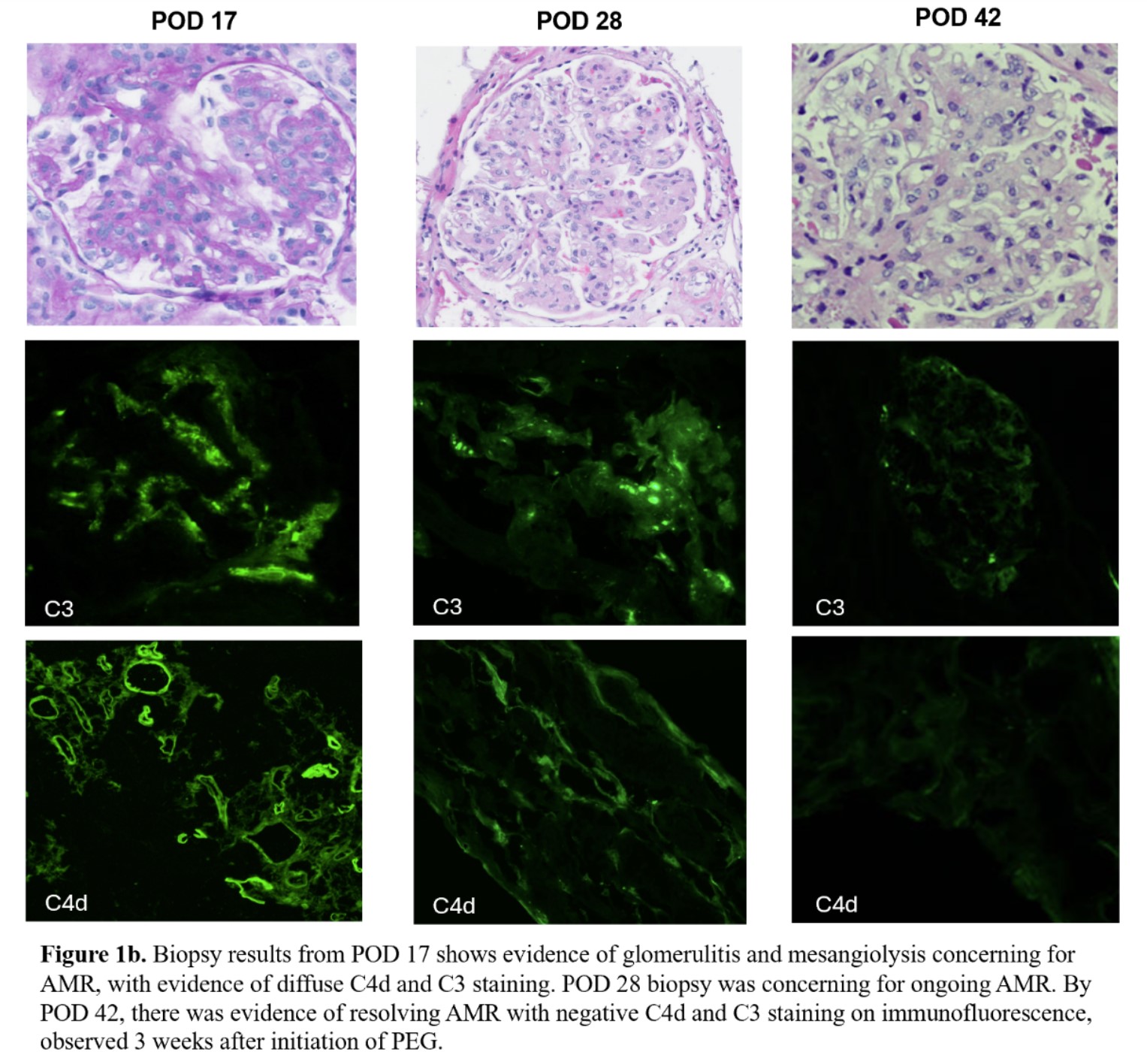
Results: There was immediate xenograft function and no evidence of clinically significant proteinuria until POD 16 (Fig 1a). A for cause biopsy was concerning for AMR (Fig 1b) prompting a course of PP and initiation of PEG. After initiation of treatment and 5 sessions of PP, sCr and proteinuria significantly improved (Fig 1a). The PEG dosing strategy, 1080 mg intravenous (IV) bolus plus 463 mg/day continuous infusion with 1080 mg IV boluses after PP, was determined by our previous successful treatment of AMR in a previous xenokidney (GGTA1 KO thymokidney) in a 2 month decedent. The patient was converted to subcutaneous administration after completion of the initial 5 PP sessions. A second course of 5 PP sessions was initiated after a POD 28 biopsy was concerning for ongoing AMR (Fig 1b). PEG 1080 mg SC three times weekly plus 1080 mg IV boluses post-PP was continued and a biopsy on POD 42 revealed resolving AMR with negative C4d and C3 staining on immunofluorescence. There was no evidence of related adverse events with PEG using the off-label dosing strategy noted above. The patient is currently 86 days post-transplant and clinically stable.
Conclusion: PEG SC plus an IV bolus dosing strategy in the setting of PP was effective for treatment of AMR in a xenokidney transplant recipient. Future studies of the pharmacokinetics of PEG and PP will be prudent to validate these clinical findings.
This data is supported by funding from United Therapeutics Corporation, PBC. Pegcetacoplan was obtained through Apellis Pharmaceuticals' Compassionate Use Program.
[1] Immunosuppression
[2] Graft Rejection
[3] Plasmapheresis