Successful somatic support of brain-dead decedents for xenotransplantation research: A systematic review and case series
Ian Jaffe1, Imad Aljabban1, Tal Eitan1, Jacqueline Kim1, Summer Viscusi1, Karen Khalil1, Jeffrey Stern1, Adam Griesemer1, Robert Montgomery1, Philip Sommer1.
1Transplant Institute, New York University, New York, NY, United States
NYU Xenotransplant Research Group.
Introduction: The brain-dead decedent model has emerged as a novel approach to support de-risked xenotransplant research under human physiologic and immunologic conditions. However, the ability to provide somatic support for a decedent beyond approximately one week has been questioned, as has the physiologic and immunologic fidelity of the model.
Methods: A systematic review (PROSPERO: CRD42023452561) was conducted by two independent reviewers and coders to identify cases in which somatic support of a brain-dead decedent had been conducted for ≥7 days. Xenotransplant decedent studies conducted after the systematic review were identified. Additionally, we report on two cases of prolonged somatic support for xenotransplant research conducted by our group. Decedent research was conducted under the approval and procedures of the NYU Research on Decedents Oversight Committee.
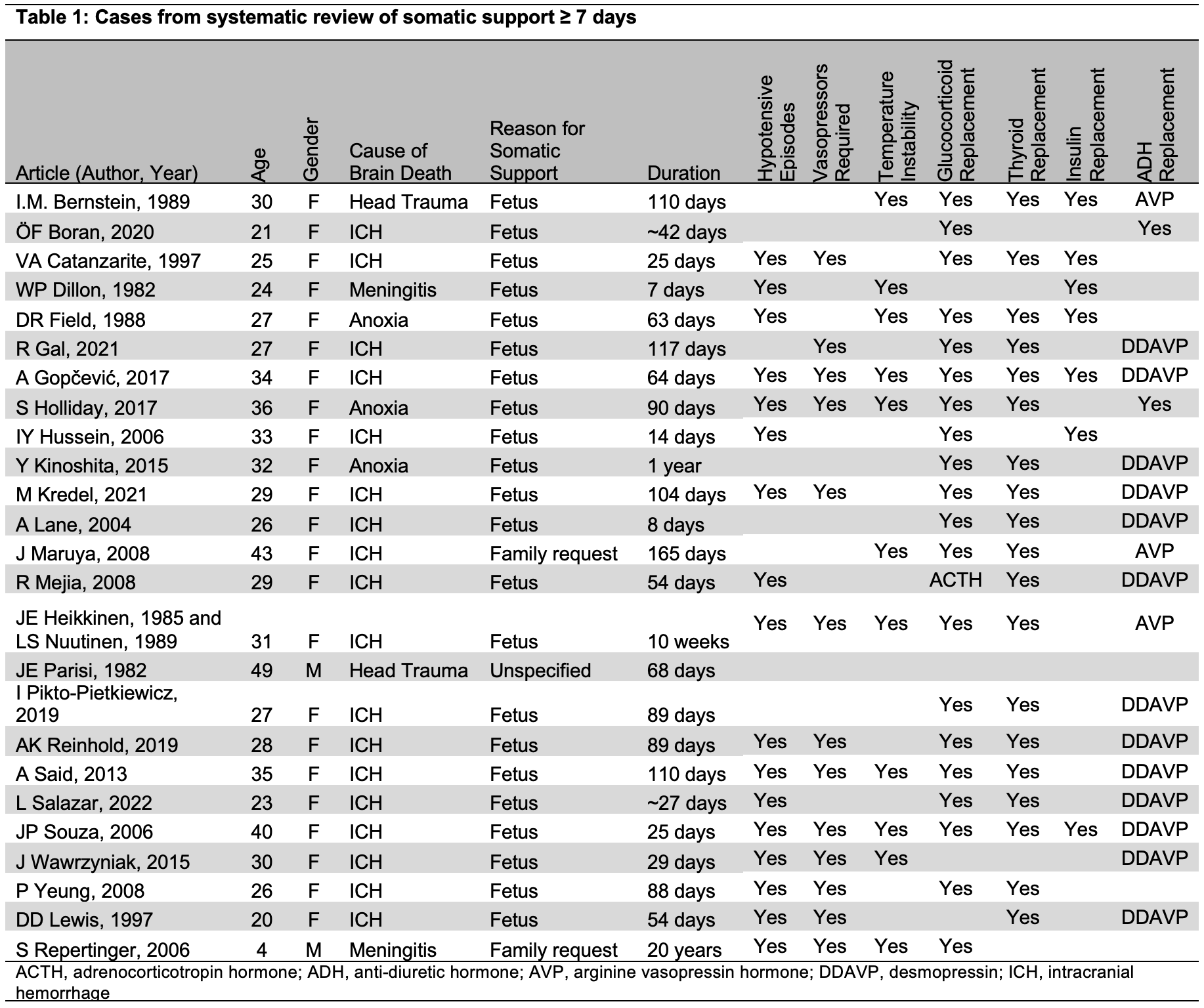
Results: For the systematic review, 26 articles reporting on 25 cases of somatic support, generally to support the gestation of a fetus (88%), were identified (Table 1). Consequently, most decedents were female (92%). Cause of death was most commonly intracerebral hemorrhage (72%). Duration of somatic support was a median of 68 days. Replacement of hypothalamic-pituitary driven hormones, the use of vasopressors for hemodynamic instability, and temperature instability were commonly reported. Since the systematic review, at least 7 cases of somatic support ≥7 days have been conducted: two by our group (providing 63 and 37 days of somatic support), as well as studies providing at least 10, 12, 12, 12, and 22 days of somatic support by groups in China and the U.S. In the two cases we performed, decedents were maintained through planned study termination points. In both cases, traditional intensive care, pharmacologic blood pressure management, and repletion of thyroid hormone (either oral or intravenous, guided by thyroid studies), anti-diuretic hormone, and glucocorticoids maintained hemodynamic stability within target mean arterial pressure range (65-100 mmHg). Clinically significant alterations in coagulation were not observed, although prolonged clot reaction time and decreased clot maximum amplitude were present at several points in both cases. Infections requiring antibiotic therapy were present in both cases as a result of conditions present at the time of death; in both cases, systemic infections were able to be cleared or controlled, even in the context of systemic immunosuppression. Hypothermia requiring active warming was present in both cases.
Conclusion: Somatic support of decedents is rare but has been successfully performed for the past few decades (typically for fetal gestation), requiring critical care, replacement of brainstem-driven hormones, and often management of hemodynamic and temperature instability. Successful somatic support for xenotransplant research has been replicated by several groups for periods of time ranging from 10-63 days. Further data on inflammatory and immunologic responses after brain death are forthcoming.
[1] decedent
[2] decedent model
[3] brain death
[4] xenotransplant
[5] pre-clinical model