Kohei Kinoshita, United States has been granted the TTS IXA Congress Scientific Awards
Retention of a native kidney with ureter ligation compensates for renin-angiotensin system function after pig-to-baboon kidney transplantation
Kohei Kinoshita1, Maho Terashita1, Akihiro Maenaka1, Ryousuke Satou2, Akemi Katsurada2, Ivy A. Rosales3, Gweneth E. Lavalla1, David Ayares4, Tatsuo Kawai1, L Gabriel Navar2, David K.C. Cooper1.
1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, Boston, MA, United States; 2Hypertension and Renal Center, Tulane University, New Orleans, LA, United States; 3Immunopathology Research Laboratory, Department of Pathology, Massachusetts General Hospital, Boston, MA, United States; 4Revivicor Inc., Blacksburg, VA, United States
Purpose: The ability of pig kidneys to fulfill physiological roles after transplantation (Tx) into baboons, including the renin-angiotensin-aldosterone system (RAAS) function, remains unclear. In prior studies where bilateral native nephrectomy was performed at pig kidney Tx (KTx), persistent dehydration required frequent saline infusions to prevent elevated serum creatinine (Cre). Additionally, recent pig-to-decedent human models with nephrectomy reported low or undetectable circulating renin. This differs from clinical KTx, where native kidneys are retained. We exlored this topic by preserving one native kidney (after ureter ligation to observe life-supporting xenograft function) during pig KTx.
Methods: Baboons (n=6) received kidneys from gene-edited pigs (n=4, triple-knockout [TKO] with growth hormone-receptor-knockout and 6 human protective proteins: hCD46, hCD55, hTBM, hEPCR, hHO1, hCD47). Two baboons underwent bilateral nephrectomy (Group A) and four retained one native kidney with ureter ligation so that it could not contribute to salt and volume excretion (Group B). Induction therapy included anti-thymocyte globulin, anti-CD20mAb, and C1-esterase inhibitor. Maintenance therapy included anti-CD154mAb, rapamycin, methylprednisolone, and IL-6R blockade with tocilizumab. Monitoring included clinical parameters and endocrine assays for RAAS in collected blood samples.
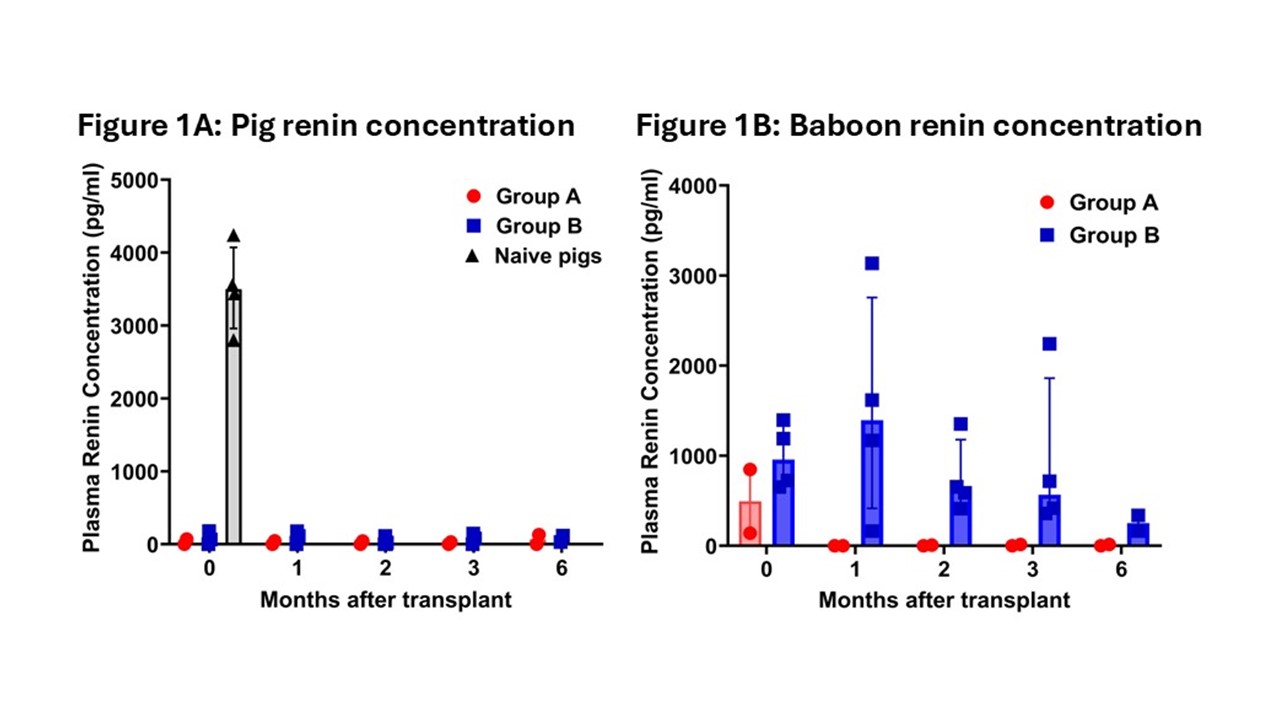
Results: The immunosuppressive regimen supported graft survival >1 year (n=2), >6 months (n=2), and >3 months (n=2). Group A exhibited features of dehydration: e.g., increasing Cre levels (requiring weekly, bi-, or tri-weekly normal saline administration) and higher potassium levels. Group B maintained normal Cre levels without fluid support. Naive pigs showed positive pig renin, but pig renin was undetectable at all post-transplant time-points in both groups (Figure 1A). Baboon renin concentration was measurable only in Group B (Figure 1B), indicating that the remaining kidney contributed to renin production. Group B showed higher baboon renin concentrations and activity than Group A, resulting in elevated angiotensin I, which indicated that angiotensinogen was cleaved by baboon renin. There were no differences in antiotensinogen or aldosterone levels between groups.
Conclusions: The absence of detectable pig renin highlights a potential physiological challenge in xenotransplantation. However, (1) retaining one native kidney with ureter ligation compensates for RAAS deficiencies observed after pig-to-baboon kidney transplantation (with bilateral native nephrectomy) and prevents dehydration. In clinical pig-to-human kidney xenotransplantation, preservation of native human kidneys may similarly maintain the RAAS with human renin. (2) Investigations need to be continued to determine whether the loss of RAAS function is related to an absence of (i) production or (ii) secretion of pig renin, or (iii) incompatibility of pig renin to interact with human angiotensinogen.
Work on xenotransplantation in our laboratory is supported in part by NIH NIAID U19 grant AI090959 and in part by a Kidney X Prize from the US DHHS and the American Society of Nephrology..
[1] Baboon
[2] kidney
[3] Pig
[4] RAAS
[5] Renin
[6] Xenotransplantation