Urinary loss of anti-CD154 therapeutic antibodies: The impact of proteinuria on pig kidney xenotransplantation
Kohei Kinoshita1, Maho Terashita1, Akihiro Maenaka1, Ivy A. Rosales2, David Ayares3, Seth Lederman4, Robert B. Colvin2, Richard N. Pierson III1, Tatsuo Kawai1, David K.C. Cooper1.
1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, Boston, MA, United States; 2Department of Pathology, Immunopathology Research Laboratory, Massachusetts General Hospital, Boston, MA, United States; 3Revivicor Inc., Blacksburg, VA, United States; 4Tonix Pharmaceuticals Holding Corp., Chatham, NJ, United States
Purpose: Proteinuria is a common complication following pig kidney xenotransplantation. It is well-documented that therapeutic antibodies can be filtered and excreted in the urine in patients with proteinuria. Although blockade of the CD40/CD154 co-stimulation pathway is widely considered the optimal immunosuppressive strategy for xenotransplantation, we report here that therapeutic antibodies targeting these pathways may be lost in the urine under conditions of significant proteinuria. This urinary loss may result in subtherapeutic antibody levels, leading to inadequate immunosuppression and graft rejection.
Methods: Nine baboons received kidneys from gene-edited pigs (either triple-knockout or double-knockout with growth hormone receptor knockout, combined with six human transgenes: hCD46, hCD55, hTBM, hEPCR, hHO-1, and hCD47; Revivicor). The immunosuppressive regimen included induction therapy with anti-thymocyte globulin, an anti-CD20mAb, and C1-esterase inhibitor, followed by maintenance therapy with an anti-CD154mAb (Tonix-1500), rapamycin, methylprednisolone, and anti-IL-6 receptor blockade (tocilizumab). All animals were confirmed cytomegalovirus (CMV)-negative. Post-transplant monitoring included blood counts, serum and urine analyses, ultrasound examinations, and renal biopsies.
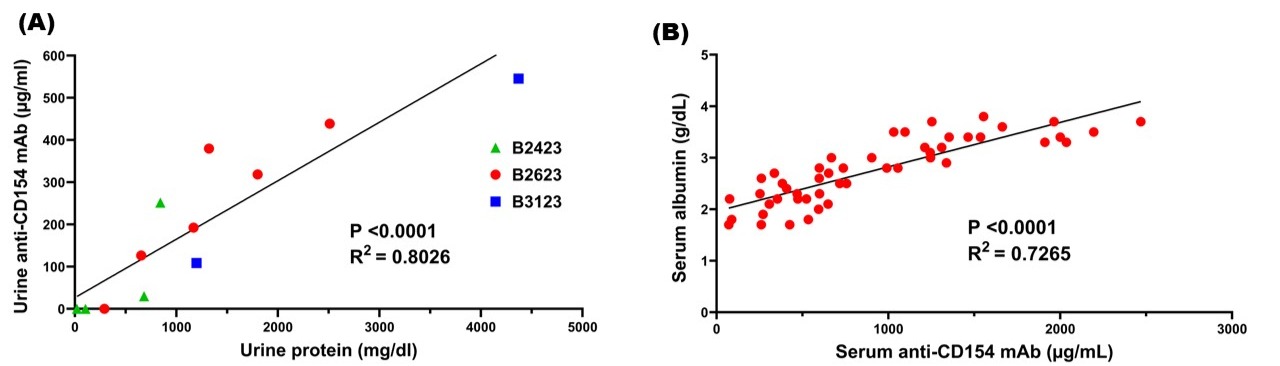
Results: Four of nine recipients who survived beyond four months developed persistent nephrotic-range proteinuria (urine protein-to-creatinine ratio >3.5g/g). However, all four maintained normal or near-normal serum creatinine levels for weeks to months. Histopathological analysis of these grafts revealed focal glomerular thrombotic microangiopathy (4/4), transplant glomerulopathy (3/4), focal segmental glomerulosclerosis (2/4), or membranous nephropathy (1/4), without classical features of xenograft rejection. Proteinuria correlated with urinary loss of therapeutic antibodies (Figure 1A), and low serum albumin levels were associated with reduced trough levels of anti-CD154mAb (Figure 1B).
Conclusions: Previously, we reported a single case of urinary loss of anti-CD154mAb associated with proteinuria. The present study expands on this finding by documenting nephrotic-range proteinuria in four baboons. We suggest that the development of nephrotic-range proteinuria remains a critical barrier to successful xenotransplantation, as it leads to the urinary loss of therapeutic immunosuppressive antibodies and increases the risk of graft rejection by inadequate immunosuppression. Elucidating the upstream mechanisms leading to proteinuria will be essential for the success of clinical pig kidney xenotransplantation.
Work on xenotransplantation in DKCC’s laboratory is supported in part by NIH NIAID U19 grant AI090959 and in part by a Kidney X Prize from the US DHHS and the American Society of Nephrology.
[1] Baboon
[2] Immunosuppressive therapy
[3] Kidney
[4] Pig
[5] Proteinuria
[6] Xenotransplantation
[7] Urinary Loss of Anti-CD154 Therapeutic Antibodies