Muhammed Esad Gunes, United States
Associate Research Scientist
Center for Translational Immunology
Columbia University
GLP preclinical trial of pig-to-baboon thymokidney xenotransplantation using inbred GalT-KO miniature swine donors
M. Esad Gunes1, Daniel Wolbrom1, Susan Qudus1, Sho Fujiwara1, Dilrukshi Ekanayake-Alper1, Kazuhiko Yamada1, Ivy Rosales2, Ibrahim Batal3, David Berglund4, Erik Berglund5, David H Sachs1, Megan Sykes1, Greg Nowak1.
1Columbia Center for Translational Immunology, Columbia University, New York, NY, United States; 2Department of Pathology, Massachusetts General Hospital, Boston, MA, United States; 3Department of Pathology, Columbia University, New York, NY, United States; 4Department of Immunology, Genetics, and Pathology, Uppsala University, Uppsala, Sweden; 5Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, Sweden
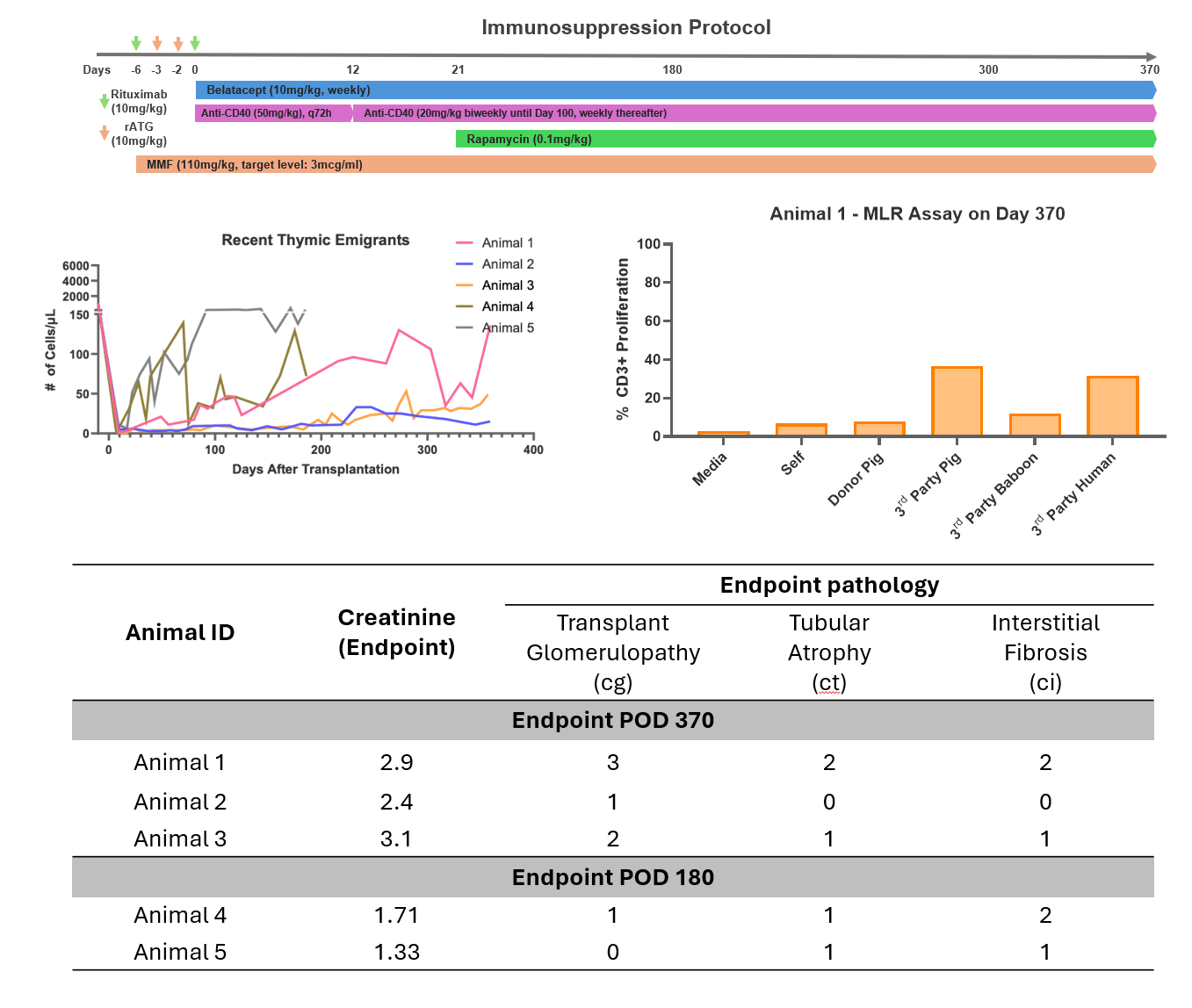
Introduction: The International Xenotransplantation Association has recommended clinical xenotransplantation trials based on preclinical studies performed under Good Laboratory Practice (GLP), in which consistent life-supporting xenograft survival of >6 months has been achieved. Here, we present the first preclinical GLP study of pig-to-baboon thymokidney (TK) xenotransplantation using single knock-out (GalT-KO) swine as donors.
Methods: Recipient baboons (n=5) with low natural non-Gal antibodies underwent total thymectomy and induction therapy with rATG and rituximab. On day 0, they underwent bilateral nephrectomy and splenectomy and received transplants of TK grafts from inbred GalT-KO miniature swine. Immunosuppression (IS) included MMF, rapamycin, anti-CD40 mAb, and Belatacept. Kidney function was assessed through biochemical analyses and biopsies. Donor-specific antibodies (DSA) and absolute T-cell, B-cell, and recent thymic emigrant (RTE) counts were monitored. Mixed lymphocyte reaction (MLR) assays were performed monthly.
Results: Two animals were followed until POD 180, and 3 animals were continued through POD 365. Mean creatinine was 1.88±0.53 and 2.8±0.29 mg/dl on days 180 and 370, respectively for the two groups. Kidney biopsies indicated mild transplant glomerulopathy and mild interstitial fibrosis without any signs of rejection or immunoglobulin deposition. After the post-transplant decrease, DSA remained stable. RTEs defined as CD45RA+CCR7+CD28+CD95-CD31+ cells, were detected in the circulation within two months after transplantation. In all animals except Animal 5, at least one MLR indicated pig-specific hyporesponsiveness.
Conclusion: In a GLP setting, we achieved 1 year life-supporting TK xenograft survival in baboons utilizing single GalT-KO miniature swine as donors. TK grafts maintained good function with no signs of rejection prior to termination. Post-transplant detection of RTE suggests that functioning pig thymus grafts may contribute to pig-specific hyporesponsiveness. Our results suggest that single GalT-KO inbred miniature swine donors may be appropriate for use in clinical trials. Future studies will focus on enhancing thymus-dependent tolerance to achieve IS-free survival.
This project was funded by NefroHealth and ChoironeX. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health, under awards S10OD030282. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Lectures by Muhammed Esad Gunes
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-01 15:05 - 15:55 |
Immunosuppression and Tolerance 1 | Immunosuppression weaning in a pig-to-baboon thymokidney xenotransplant model | Auditorium |
|
Thu-02 16:20 - 17:05 |
Pre- and Subclinical Models 2 | GLP preclinical trial of pig-to-baboon thymokidney xenotransplantation using inbred GalT-KO miniature swine donors | Auditorium |
