Muhammed Esad Gunes, United States
Associate Research Scientist
Center for Translational Immunology
Columbia University
Immunosuppression weaning in a pig-to-baboon thymokidney xenotransplant model
M. Esad Gunes1, Sho Fujiwara1, Daniel Wolbrom1, Susan Qudus1, Mahak Masoodi1, Lara Badr1, Ivy Rosales2, Ibrahim Batal3, David Berglund4, Erik Berglund5, David H Sachs1, Megan Sykes1, Greg Nowak1.
1Columbia Center for Translational Immunology, Columbia University, New York, NY, United States; 2Department of Pathology, Massachusetts General Hospital, Boston, MA, United States; 3Department of Pathology, Columbia University, New York, NY, United States; 4Department of Immunology, Genetics, and Pathology, Uppsala University, Uppsala, Sweden; 5Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, Sweden
Introduction. Chronic rejection and immunosuppression (ISP)- associated toxicity and infection are persistent obstacles to successful xenotransplantation. The ultimate solution could be the induction of tolerance. This study investigated thymus-dependent tolerance induction in a pig-to-baboon thymokidney (TK) xenotransplantation model using GalT-KO inbred miniature swine.
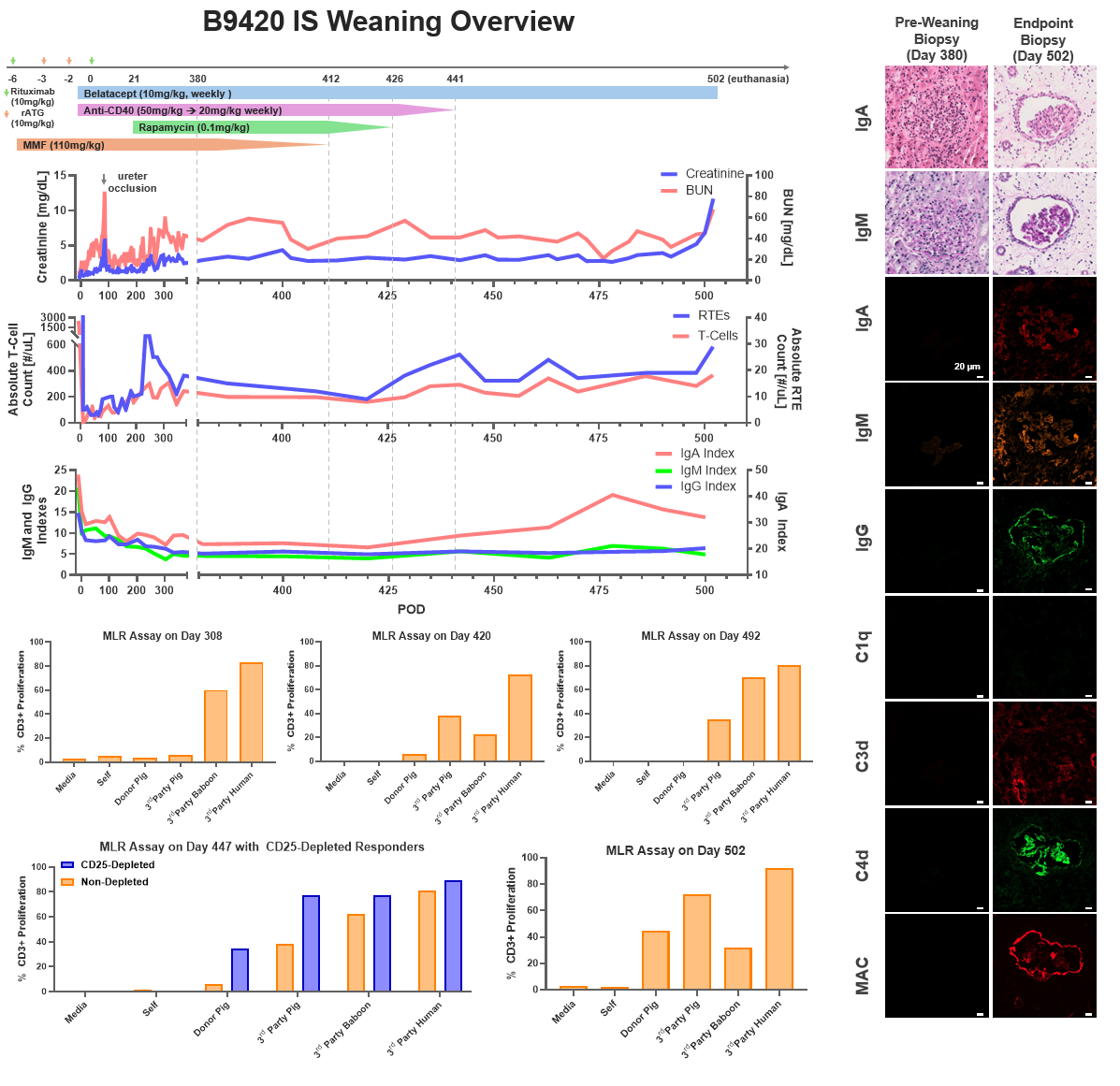
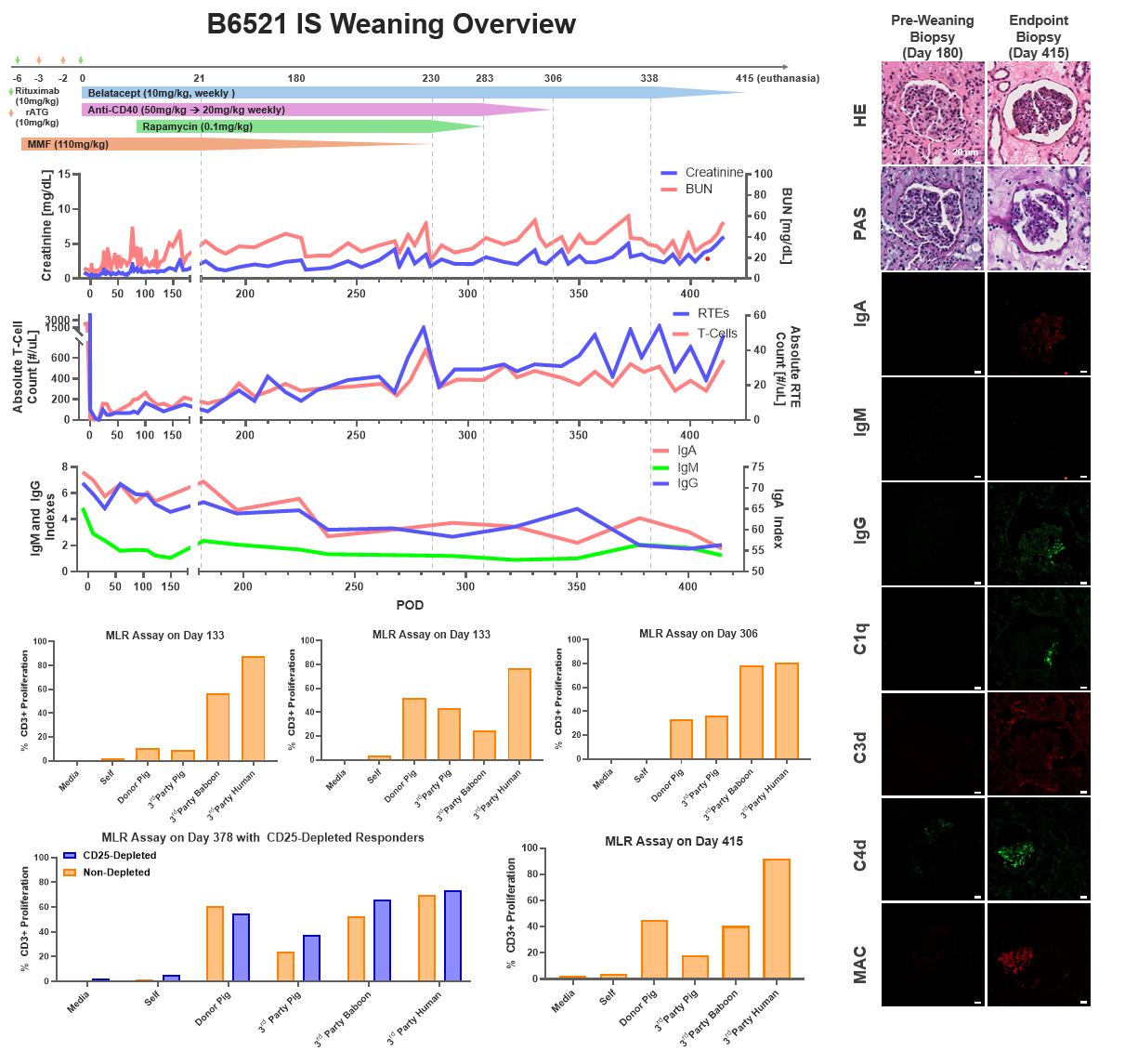
Methods. GalT-KO SLAhh inbred miniature swine were used as the TK donor animals. The recipient baboons (n=2, B9420 and B6521) underwent total thymectomy and induction therapy with rATG and rituximab. On day 0, they underwent bilateral nephrectomy, splenectomy, and received a life-supporting TK graft. Post-transplant ISP consisted of MMF, anti-CD40 mAb, rapamycin, and belatacept. ISP was gradually weaned after post-transplant day 180 in B6521 and day 380 in B9420. Protocol graft biopsies were performed. Donor-specific antibodies (DSA), absolute T-cell, B-cell, and recent thymic emigrant (RTE) counts were monitored. Mixed lymphocyte reaction (MLR) assays were performed to monitor tolerance development.
Results. Both baboons recovered well from the surgery and maintained a stable clinical course until ISP weaning. RTEs, defined as CD45RA+CCR7+CD28+CD95-CD31+, started to appear 2 months after the transplant (peaking at 33 cells/µL for B9420 and at 55 cells/µL for B6521). After day 120, consecutive MLR assays indicated pig-specific hyporesponsiveness in both animals. Following 180 days (B6521) and 380 days (B9420) of stable graft function, ISP was gradually weaned. Both animals maintained stable renal functions after withdrawal of MMF, rapamycin, and anti-CD40 mAb. After 61 days (B9420) and 77 days (B6521) of subsequent belatacept monotherapy, the kidney function rapidly deteriorated in both, B6521 and B9420, and the animals were euthanized on days 415 and 502, respectively. B9420 maintained its hyporesponsiveness until the endpoint MLR, while B6521 recovered anti-donor responses during weaning of IS. In B9420, CD25-depletion MLR indicated that hyporesponsiveness was Treg-mediated. IgM and IgG DSA remained unchanged for the duration of the study, while IgA DSA increased 2-fold in B9420 during monotherapy with Belatacept. Before ISP weaning, biopsies in both animals showed mild transplant glomerulopathy and fibrosis with no sign of rejection or immunoglobulin and complement deposition. In contrast, biopsy at the time of euthanasia in both animals showed acute T-cell and chronic antibody-mediated rejection with IgA, IgM, IgG, C4d, and MAC deposition.
Conclusion. Donor-specific hyporesponsiveness was achieved in thymokidney xenotransplantation from a GalT-KO SLAhh swine donor. Although clinical tolerance was not achieved, the kidney grafts survived >1 year, including >2 months on belatacept monotherapy in both animals. Our study suggests that ISP can be markedly reduced but not fully discontinued after TK xenotransplantation, in association with donor-specific hyporesponsiveness.
This project was funded by NefroHealth and ChoironeX. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health, under awards S10OD030282. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health..
Lectures by Muhammed Esad Gunes
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-01 15:05 - 15:55 |
Immunosuppression and Tolerance 1 | Immunosuppression weaning in a pig-to-baboon thymokidney xenotransplant model | Auditorium |
|
Thu-02 16:20 - 17:05 |
Pre- and Subclinical Models 2 | GLP preclinical trial of pig-to-baboon thymokidney xenotransplantation using inbred GalT-KO miniature swine donors | Auditorium |
