Postdoctoral fellow
PARCC - Inserm U970
Paris Institute for Transplantation and Organ Regeneration (PITOR)
Spatial multimodal characterization of long-term immune response after pig-to-human kidney xenotransplantation
Valentin Goutaudier1, Erwan Morgand1, Jeffrey Stern2, Fariza Mezine1, Karen Khalil2, Claire Williams3, Idris Boudhabhay4, Lubka Roumenina4, Aurélie Sannier1, Aprajita Mattoo2, Vasishta Tatapudi2, David Ayares5, Patrick Bruneval1, Adam Griesemer2, Massimo Mangiola2, Robert A. Montgomery2, Alexandre Loupy1.
1Paris Institute for Transplantation and Organ Regeneration (PITOR), PARCC - Inserm U970, Paris, France; 2NYU Langone Transplant Institute, New York University, New York, NY, United States; 3Spatial Biology Department, Bruker Corporation, Billerica, MA, United States; 4Cordeliers Research Center, Inserm, Paris, France; 5Research Triangle Park, United Therapeutics Corporation, Durham, NC, United States
Introduction: We previously characterized the short-term immune response after pig-to-human kidney xenotransplantation, but little is known about its long-term evolution. Here we aimed to decipher the long-term xenoimmune response in a human decedent model.
Methods: We analyzed a genetically modified pig kidney xenograft implanted for 61 days in a deceased human recipient with a pre-transplant negative xenoreactive crossmatch. We performed morphological evaluation, multi-immunophenotyping, 38-hyperplex complement cascade imaging, bulk transcriptomic profiling, and spatial transcriptomics at cellular and subcellular levels (NanoString CosMx®) at sequential time points (POD10, POD33, POD45, POD49, POD61). Controls included pre-implantation xenograft, wild-type pig kidney autografts, and brain-dead pig kidneys.
Results: From POD10 to POD61, we observed a membranoproliferative glomerulonephritis (MPGN)-like pattern characterized by microvascular inflammation, predominantly in glomeruli, associated with intense linear capillary C4d deposition, glomerular deposition of IgM and IgA, endothelial activation, and early post-xenotransplant de novo positive xenoreactive crossmatch. This pattern was accompanied by glomerular double contours, interstitial edema and endarteritis from POD45. Capillary inflammation primarily consisted of CD68+ and CD15+ innate immune cells throughout all follow-up time points. At POD45, we observed co-localized deposits of IgM, IgA, C1q, C3c, C4d, factor B, and complement factor H regulator along glomerular capillaries. The xenograft exhibited increased expression of genes related to humoral response, including monocyte and macrophage activation, NK cell burden, endothelial activation, interferon-gamma response, and T and B cell interaction, which improved over time after rejection treatment (Figure).
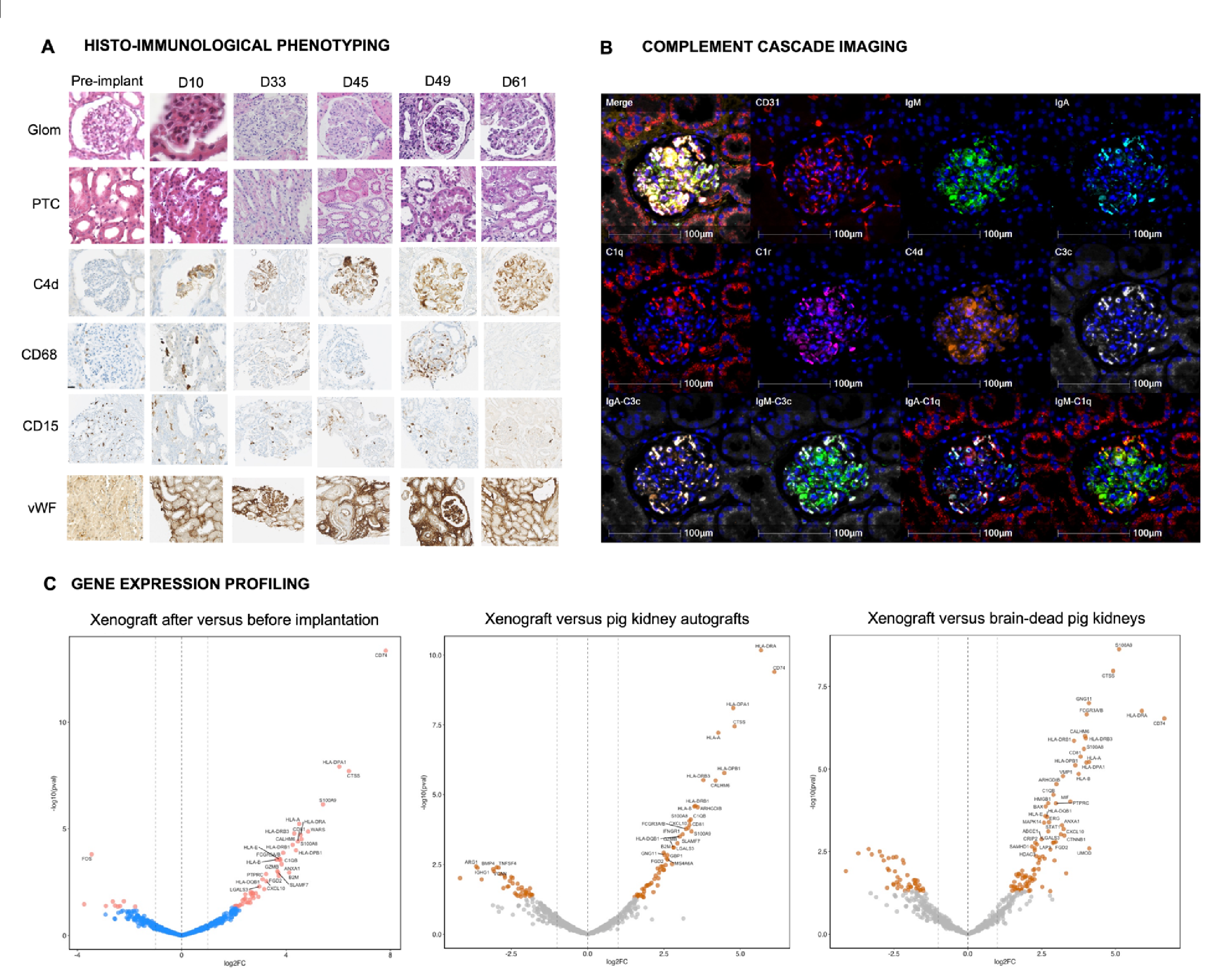
Conclusion: We described for the first time a new entity of xenoantibody-mediated injury characterized by an MPGN-like pattern, mainly driven by complement activation through glomerular xenoimmune complexes, which improved after rejection treatment. These findings support the development of personalized therapies to improve xenotransplantation outcomes.
References:
[1] Xenograft Rejection
[2] Multimodal Phenotyping
[3] Xenograft Pathology
[4] Transcriptomic Profiling
[5] Precision Medicine
[6] Xenoimmune Response
Lectures by Valentin Goutaudier
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-02 15:05 - 15:55 |
Innate Immunity and Inflammation 1 | Spatial multimodal characterization of long-term immune response after pig-to-human kidney xenotransplantation | H8-01-F |
|
Thu-02 16:20 - 17:05 |
Innate Immunity and Inflammation 2 | Application of a novel spatial transcriptomic 6000-plex panel in pig-to-human xenotransplantation | H8-01-F |
