Quality systems driving innovation: Implementing QMS, QA, and QC for genetically modified pigs in xenotransplantation
Ludmila Garcia1, Gabrielli Vaz Sampaio1, André Costa1, Luiz Gustavo Cano Munhoz1, Michelle Silva Araujo1, Vitor Leão1, Ligiane de Oliveira Leme1, Luciano Brito1, Mayana Zatz1,2, Silvano M. A. Raia1, Ernesto Goulart1,2.
1Quality Assurance - Xeno Brasil, Sao Paulo University, São Paulo , Brazil; 2Genetics and evolutionary biology, Sao Paulo University, São Paulo, Brazil
Xenotransplantation is emerging as a transformative solution to the global organ shortage. However, the path from laboratory innovation to clinical application requires not only robust genetic engineering, but also rigorous implementation of quality systems. This study presents the experience of XenoBr, a Brazilian biotech start-up, in establishing and operationalising a comprehensive quality management system (QMS) to ensure the safety, reliability and regulatory compliance of genetically modified porcine donors for xenotransplantation.
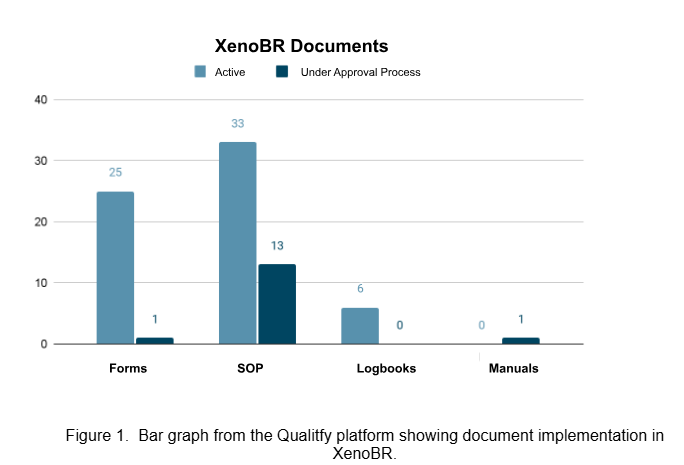
A dual framework integrating quality assurance (QA) and quality control (QC) was implemented under GMP-aligned workflows. The QA strategy focused on structuring internal processes through standardised documentation, training and an online document and event management platform (Qualitfy) in line with ISO 9001 standards. A total of 64 documents - including SOPs, logbooks and traceability forms - were approved and implemented. QA also led the development of a Good Laboratory Practices (GLP) manual, which is now central to team training and audit readiness.
In parallel, QC activities focused on biosafety, genetic stability and microbial contamination. Microbiological monitoring used validated ATP bioluminescence assays (MicroSnap®) and mycoplasma detection (MycoAlert®) with confirmatory DAPI staining and PCR testing.
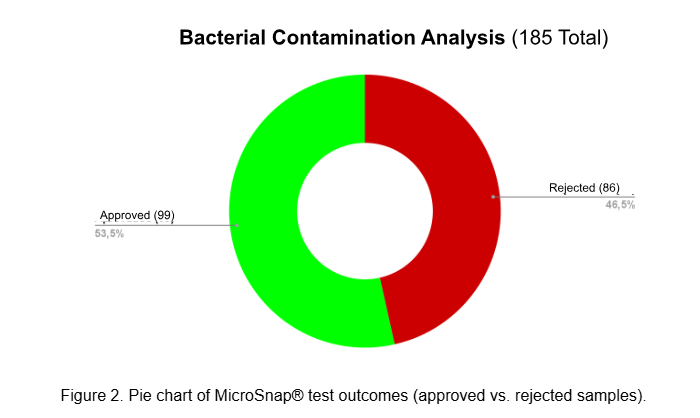
While the majority of samples met safety thresholds (CFU <10; B/A <0.9), a subset revealed contamination (e.g. Staphylococcus coagulase negativa, Micrococcus spp. and elevated ATP signals on laboratory surfaces), prompting immediate corrective actions including retraining and protocol revision.
In addition, a cytogenetic karyotype test was standardised to assess chromosomal integrity, with initial results confirming the presence of sex chromosomes and supporting the genomic stability of porcine fibroblast lines. Challenges such as early phase contamination underlined the importance of rigorous QA-QC interplay for data validity.
São Paulo Research Foundation (FAPESP) - grant number 21/11872-5. National Council for Scientific and Technological Development (CNPq) - grant number 444075/2023-2.
[1] Xenotransplantation
[2] Genetically Modified Pigs
[3] Quality Management System, QA, QC,
[4] Biosafety
[5] GMP
[6] Regulatory Compliance