Chung Young Kim, Korea has been granted the TTS IXA Congress Scientific Awards
Enhanced graft survival in full-thickness corneal xenotransplantation from glycan or complement gene-edited pigs to non-human primates
Chung Young Kim1,2, Dong Hee Choi2, Jin Suk Ryu2, Won Young Jeong3, Jong-Min Kim3,4, Sang-Ik Cho5, Eun-Jee Oh6, Chang Ho Yoon1,2, Chung-Gyu Park3,7, Joohyun Shim8, Hyunil Kim8, Mee Kum Kim1,2,3.
1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea; 2Laboratory of Ocular Regenerative Medicine and Immunology, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea; 3Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea; 4Department of Animal Health and Welfare, College of Health and Medical Science, Cheongju University, Cheongju, Korea; 5Department of Medical Sciences, Graduate School of The Catholic University of Korea, Seoul, Korea; 6Department of Laboratory Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea; 7Department of Microbiology and Immunology, Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea; 8Optipharm, Inc., Cheongju, Korea
Introduction: Given the persistent shortage of donor corneas in East Asia, porcine corneas are being explored as alternative graft sources. While early genetically engineered (GE) models demonstrated limited graft survival without substantial immunosuppression, recent multi-gene-edited pigs are being developed to overcome these limitations. This study aimed to evaluate the safety and efficacy of full-thickness corneal xenotransplantation from pigs with targeted disruption of four glycan-related genes—GGTA1, CMAH, β4GalNT2, and iGb3s (QKO)—alongside transgenic expression of two human immune-regulatory genes, CD55 and CD39.
Methods: The QKO genotype was designed to eliminate key xenoantigens such as Galα(1,3)Gal, Neu5Gc, β4GalNT2, and iGb3s. To further improve immune modulation, hCD55 and hCD39 were introduced to inhibit complement activation and regulate inflammatory signaling. Full-thickness corneas from either QKO or QKO.hCD55.hCD39 knock-in (KI) pigs were transplanted into six cynomolgus monkeys (two received QKO grafts, four received QKO.hCD55.hCD39 grafts). Postoperatively, systemic and topical immunosuppressive regimens were administered. Ocular assessments included rejection index, central corneal thickness, intraocular pressure, and C3a levels in aqueous humor. Systemic monitoring included body weight/temperature, CBC, renal and liver function tests, tacrolimus trough levels, T/B cell analysis (flow cytometry), and donor-specific IgG/IgM (ELISA)
Results: QKO grafts survived for 10 and 14 weeks, respectively. One case experienced rejection two weeks after tacrolimus withdrawal due to weight loss. In both cases, highly increased levels of IFN-γ–secreting CD8+ T cells were detected one week before rejection. Concurrently, donor-specific IgG/IgM antibodies and aqueous humor C3a levels were significantly elevated at the time of rejection. In contrast, QKO.hCD55.hCD39 KI grafts demonstrated faster resolution of corneal edema and survived without rejection for up to 12 months. Although IFN-γ–producing CD4+/CD8+ T cells and memory T cell subset concentrations increased up to 6 months post-transplantation, levels of those cell concentration were not high compared with those of rejected group and immune activity substantially declined thereafter. Concentration of donor-specific IgG and IgM antibodies also increased during the first six months, but showed individual variation beyond that period. Aqueous humor C3a levels were well controlled.
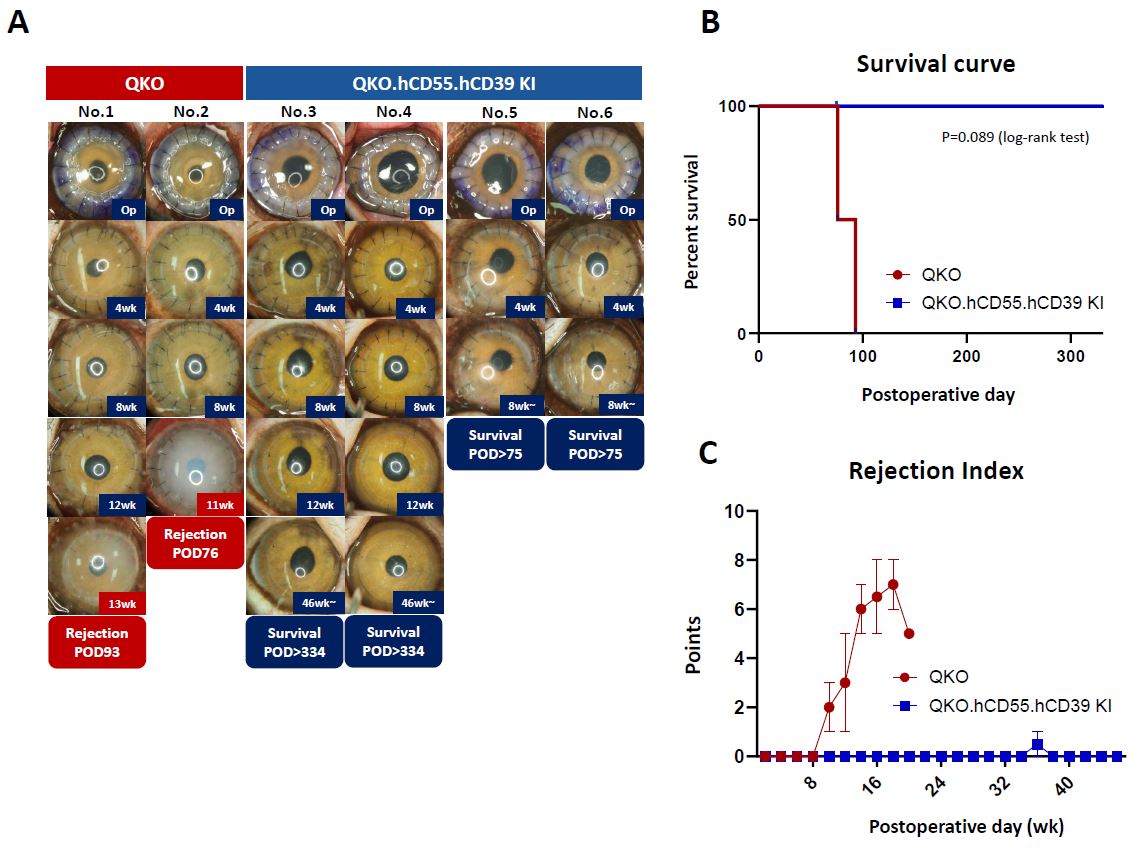
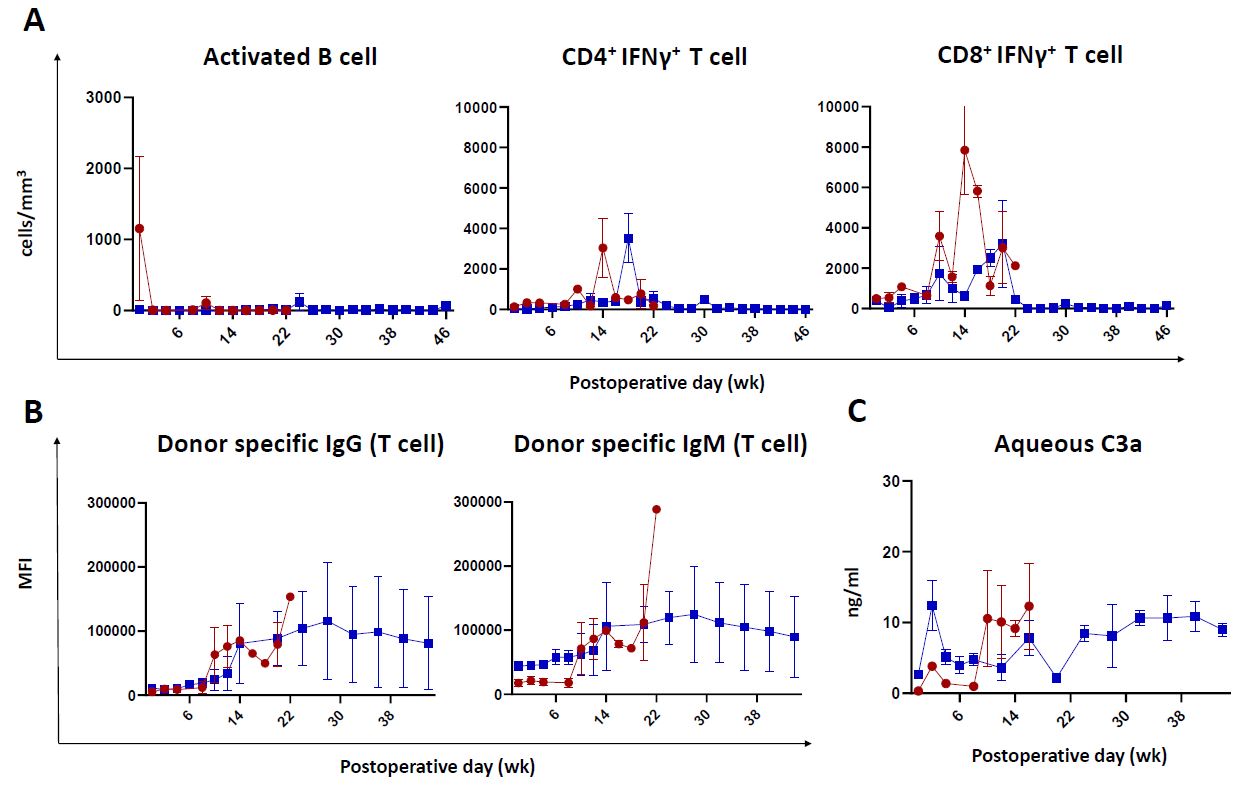
Conclusion: QKO.hCD55.hCD39 KI corneas showed significantly improved biocompatibility and prolonged graft survival compared to QKO controls. Transgenic expression of hCD55 and hCD39 effectively enhanced immune regulation and reduced rejection in corneal xenotransplantation. Systemic T and B cell suppression was critical for the first 6 months post-transplantation, and ongoing B cell control beyond this period should be individualized based on recipient immune profiles.