Human xenoreactive antibodies against porcine aortic endothelial cells
Imad Aljabban1, Jacqueline Kim1, Ian Jaffe1, Karen Khalil1, Tal Eitan1, Amy Dandro2, Ana Fazio-Kroll2, David Ayares2, Simon Williams1, Brendan Keating1, Ming Wu3, Aprajita Mattoo1, Vasishta Tatapudi1, Edward Skolnik1, Jeffrey M Stern1, Adam Griesemer1, Robert A Montgomery1, Massimo Mangiola1.
1NYU Langone Transplant Institute, New York, NY, United States; 2United Therapeutics Corporation, Durham, NC, United States; 3Northwell Health, New York, NY, United States
Introduction: A barrier to successful xenotransplantation is the presence of donor specific antibodies that could initiate an antibody mediated response. Pre-operative crossmatch testing is a valuable technique for detecting the presence of such xenoreactive antibodies, and can inform strategies to mitigate the risk of rejection. Here, we evaluate human anti-pig reactivity against porcine aortic endothelial cells (pAECs) in a pig-to-human decedent xenotransplant model.
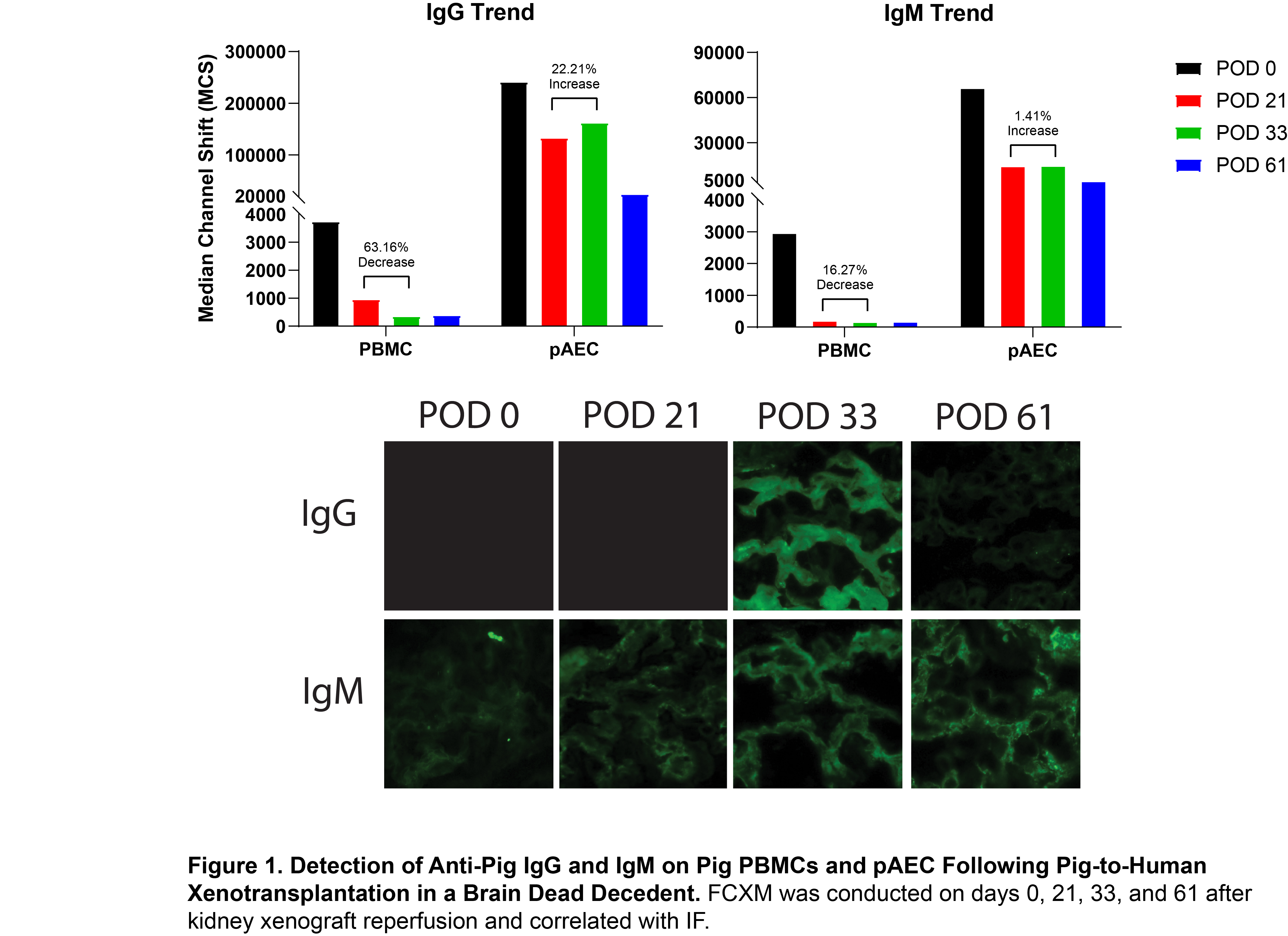
Methods: A brain-dead decedent underwent bilateral native nephrectomies followed by transplantation of a GGTA1 KO Thymokidney, with clinically approved immunosuppression administered for a planned 61-day period. Undiluted, heat-inactivated serum samples from the decedent at various timepoints were incubated with either porcine peripheral blood mononuclear cells (PBMCs) or pAECs. Human anti-pig antibody reactivity was assessed via flow cytometric crossmatch (FCXM) and quantified by measuring the median channel shift (MCS) in fluorescence intensity. Serial xenograft biopsies were collected and analyzed by immunofluorescence (IF) to detect glomerular deposition of human IgG and IgM.
Results: Mean IgG and IgM reactivity against pAECs was approximately 100-fold and 28-fold higher, respectively, compared to PBMCs. By post-operative day (POD) 33, early antibody-mediated rejection was identified, coinciding with a rise in serum creatinine (data not shown). Flow cytometric crossmatch (FXCM) using PBMCs demonstrated a 61.16% decrease in IgG and a 16.27% decrease in IgM reactivity. In contrast, FXCM with pAECs showed a 22.1% increase in IgG and a 1.41% increase in IgM reactivity (Figure 1A). These changes in anti-pig IgG and IgM responses correlated with increased glomerular deposition of IgG and IgM on immunofluorescence at POD 33 (Figure 1B). Following initiation of anti-rejection therapy, the IgG immunofluorescence signal decreased.
Conclusions: FCXM using pAECs revealed significantly higher anti-pig IgG and IgM reactivity compared to PBMCs, suggesting the presence of antigenic targets on pAECs that are either absent or expressed at lower density on PBMCs. Crossmatch on pAECs may provide a more reliable means of detecting xenoantibody mediated responses compared to PBMCs.
Work funded in part by Lung Biotechnology, a wholly owned subsidiary of United Therapeutics Corporation.
[1] Xenotransplantation
[2] Crossmatch
[3] Antibody