Cardiac xenograft as a bridge to allotransplantation in a non-human primate model: Lessons learned
William Swicord1, J H Simmons2, Sarah J Neal2, Clementine Vo3, Kanwarpal Bakshi3, Julie Juliani4, Julie Fenske4, Kristen Getchell5, Isabela Moreno5, Vincent Yeung5, David C Cleveland1, John D Cleveland1.
1Division of Cardiac Surgery, Children’s Hospital Los Angeles, Los Angeles, CA, United States; 2Michale E. Keeling Center for Comparative Medicine, MD Anderson Cancer Center, Bastrop, TX, United States; 3Division of Pediatric Cardiac Anesthesiology, Children’s Hospital Los Angeles, Los Angeles, CA, United States; 4Division of Cardiac Perfusion, University of Southern California, Los Angeles, CA, United States; 5eGenesis Biotechnology, Cambridge, MA, United States
Objective(s): Xenotransplantation of genetically engineered porcine hearts (GEPH) has been utilized as destination therapy in adult human subjects under FDA expanded access criteria. We envision this technology can save the lives of human infants with critical congenital heart disease who are currently dying on the cardiac allotransplant waiting list. A successful animal model is necessary to study this application and prove concept.
Methods: Three olive baboons aged 29, 15, and 29 months and weighing 6.9, 4.8, and 6.1 kg respectively underwent orthotopic cardiac xenotransplantation from size matched Yucatan miniature swine. Animals were maintained on immunosuppression (IS) centered on CD40 ligand blockade (Tegoprubart, Eledon Pharmaceuticals) for up to 8 months. Function of the xenograft was tracked on weekly echocardiogram and invasive hemodynamic monitoring. Immunologic responses were followed with flow cytometry. Xenografts were removed and surgically replaced with allografts from prospectively crossmatched, ABO compatible, size-matched donor baboons. Immunosuppression following this was transitioned to tacrolimus and mycophenolate mofetil. Functional and immunologic outcomes following allotransplantation were monitored in the aforementioned fashion.
Results: All animals survived >4 months with xenografts that exhibited normal systolic function up to the time of explant. There was no evidence of anti-pig antibody above pre-transplant levels nor was there evidence of same species sensitization (Figure 1). One out of three survived the allotransplant operation. The first animal was allowed to retain a small portion of porcine inferior vena cava (IVC) to facilitate repeat transplant. The second succumbed following a surgical error made in attempt to remove all porcine tissue. The third succumbed to volume overload during induction therapy prior to allotransplantation. Baboon #1 developed acute rejection 71 days following allotransplant and survived 105 days before elective euthanasia. Anti-pig antibodies increased in the setting of conventional immunosuppression and retained pig IVC.
Conclusion: Herein is proof of concept that bridge to cardiac allotransplantation with a cardiac xenograft is feasible. Prolonged exposure to GEPH does not cause same species sensitization, but conventional immunosuppression is not capable of preventing reactivity to xeno-antigens. All pig tissue must be removed prior to transition to conventional IS regimens and surgical strategies must be tailored to allow for this. Further study is warranted in the animal model.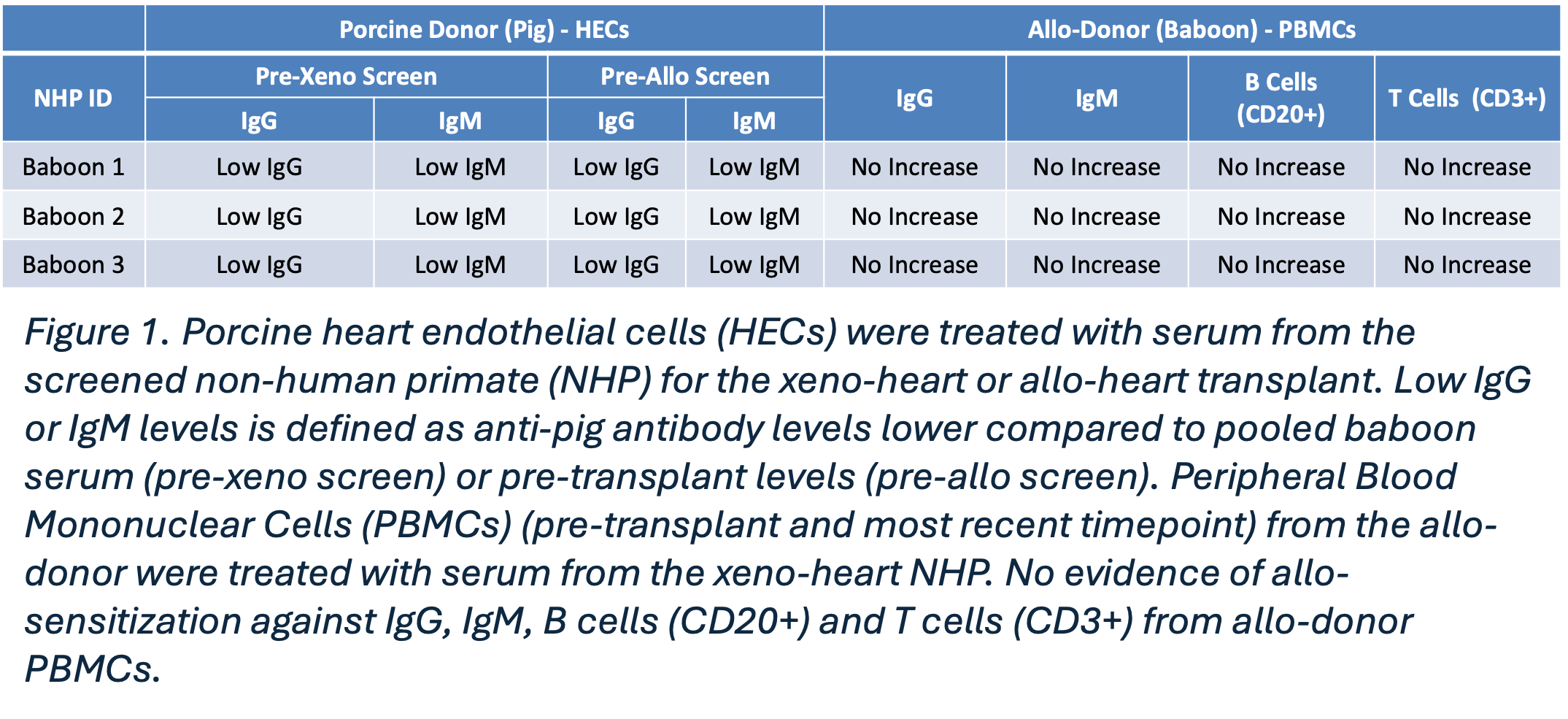
[1] Xenotransplantation