Neutrophil Extracellular Trap (NET) formation after xenotransplantation: A potential contributor to inflammation and thrombodysregulation
Amir Sanatkar1, Megan Dufault1, Sho Takemoto1, Felix Ellett4, Kirandeep K. Gill4, Sunil Poudel1, Lindemberg Da Mota Silveira Filho1, Victoria Diaz1, Jonathan Schulz1, Zahra Habibabady1, Kristin M. Whitworth2, Lars Burdorf3, William Eyestone3, David Ayers3, Richard N. Pierson1.
1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States; 2National Swine Resource and Research Center (NSRRC), , Animal Science Research Center, University of Missouri, , Columbia, MO, United States; 3Revivicor, Inc., , Blacksburg, VA, United States; 4Center for Engineering in Medicine and Surgery, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Introduction: Platelet and neutrophil sequestration immediately following reperfusion of gene-modified pig organs remains a significant challenge in xenotransplantation, particularly in lung and liver grafts. Evidence suggests that these events contribute to graft dysfunction. Neutrophil Extracellular Traps (NETs)—web-like structures of DNA and proteolytic enzymes released by activated neutrophils—play a critical role in exacerbating thrombodysregulation and cell sequestration in association with systemic infection and ischemia/reperfusion injury. We asked whether xenotransplantation is associated with NET formation in several organ xenograft models.
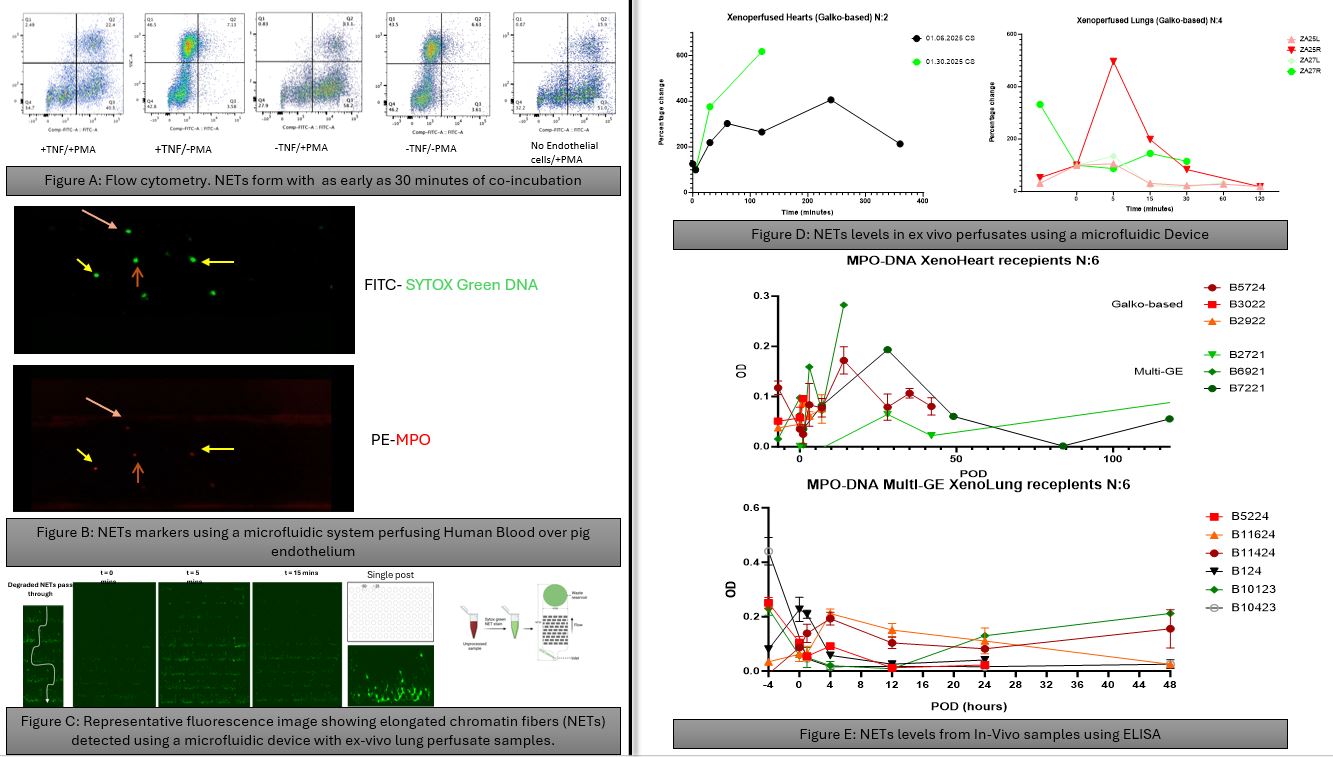
Methods: Because detecting NETs is challenging due to their transient nature, we employed multiple complementary approaches: 1) dual neutrophil positivity for DNA and neutrophil elastase by flow cytometry; 2) co-localized DNA and myeloperoxidase (MPO) binding to leukocytes during Bioflux ex vivo microfluidic ‘vascular’ perfusion; 3) mechanically trap chromatin fibers from NETs using perfusates (blood) collected during various timepoints of ex-vivo Lung perfusions Using an established microfluid device; 4) sandwich ELISA for DNA-MPO complexes in plasma from in vivo heart and lung xenograft recipients.
Results: Human buffy coat leukocytes co-incubated with wild-type (WT) pig endothelial cells (ECs) at a 1:10 ratio demonstrated neutrophil activation followed by NET formation within 30 minutes (Fig A). TNF-α (10 ng/mL) preactivation of either porcine ECs or human neutrophils significantly enhanced NET formation during Bioflux perfusion (Fig B), reaching maximal levels when both ECs and neutrophils were pre-activated. Ex vivo perfusion of pig lungs with Galko-based genetic modifications showed a rapid NET release pattern within minutes (Fig C). Ex vivo perfusion of pig hearts (N=2) or lungs (n=4) exhibited variable amounts of NET formation and kinetics (Fig D). In vivo heart and lung xenotransplant studies revealed a rise in NET markers associated with conditioning regimens used (before and at T0); within hours after graft revascularization; and in association with clinical events such as during rejection or infectious episodes.
Conclusion: Under experimental conditions where a variety of strategies aimed at reducing neutrophil overactivation were consistently deployed—including anti-platelet agents, E- and P-selectin blockade, and IL-8 inhibition—NET formation is often detectable. We speculate that NET formation may represent a key contributor to early proinflammatory and thrombodysregulatory events observed after xenotransplantation using multiply gene-edited organs. Ongoing efforts are focused on elucidating the underlying mechanisms of NET induction in this setting, particularly exploring the interactions of neutrophils with activated platelets, and identifying effective therapeutic strategies to prevent or mitigate NET-associated complications.
[1] Nuetrophils
[2] Extracellular Traps
[3] Gene modification
[4] Innate immunity
[5] Xeno Heart
[6] Xeno Lung