Establishing correlation of non-invasive monitoring techniques in xenotransplantation
Sarah Cipriano1, Muhammed Mohiuddin3, Andrew Tully2, Javier Galindo3, Susie Hong1, Marco Oldsman1, Albert Hicks1, Timm Dickfeld1, Anuj Gupta1, Alison Grazioli2,3, Allison Lankford2, Erika Feller4, Bartley Griffith2, Manjula Ananthram1.
1Cardiology, Univeristy of Maryland Medical Center, Baltimore, MD, United States; 2Cardiac Surgery, Univeristy of Maryland Medical Center, Baltimore, MD, United States; 3Medicine, Univeristy of Maryland Medical Center, Baltimore, MD, United States; 4Cardiology, Medstar Health, Baltimore, MD, United States
Introduction: Non-invasive surveillance of cardiac allograft health has included biomarkers such as NT-proBNP, and donor-derived cell-free DNA (dd-cfDNA). It has previously been shown that these biomarkers when elevated, indicate graft complications such as rejection, coronary artery vasculopathy, or donor-specific antibody production. As xenotransplantation enters the arena as a potential option for those with advanced heart failure, noninvasive monitoring techniques need to be investigated. Our institution performed the second porcine to human cardiac xenotransplant with a 10 gene edited porcine cardiac xenograft under FDA compassionate use authorization. After requiring perioperative transfusions, by post operative day (POD) 13, endomyocardial biopsy (EMB) revealed antibody and complement deposition without damage. Hemodynamic decompensation on POD 29 prompted EMB, revealing antibody mediated rejection (AMR).
Research Questions/Goals: The authors sought to investigate if NT-proBNP and dd-cfDNA positively correlate in the patient with cardiac xenograft.
Methods/Approach: NT-proBNP was checked periodically and dd-cfDNA was assessed once to twice weekly post-transplant. A retrospective review was completed including Pearson’s correlation coefficient analysis.
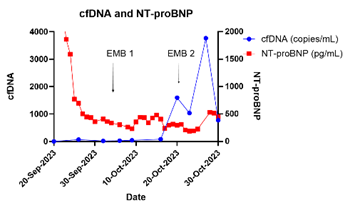
Results/Data: There was a weak negative correlation between the NT-proBNP and dd-cfDNA (r=-0.28). After the initial spike in the perioperative period, NT ProBNP levels declined. NT Pro-BNP did not rise as dd-cfDNA did at the time of biopsy proven antibody mediated rejection (AMR). See the graphic below for details.
Conclusion: Noninvasive surveillance of cardiac xenografts needs further investigation. Biomarkers such as NT-proBNP negatively correlated with dd-cfDNA, which increased as expected at the time of biopsy proven antibody mediated rejection. Porcine xenografts are accustomed to a low-pressure circulatory system in contrast to the human recipient milieu. Despite this increased load to the xenograft, NT-proBNP levels decreased significantly after the initial postoperative phase. The NT proBNP values could have been lower than expected due to the effects of continuous renal replacement therapy. It is interesting to also note that the human assay detected xeno NT-proBNP.
The source animal was provided by Revivicor Inc., and the Tegoprubart antibody was provided by Eledon Pharmaceuticals, both in kind. CareDx and Karius analytics were provided in kind. United Therapeutics, Inc. provided funding to the University of Maryland Foundation to help defray the cost of this transplant. No financial support was provided by Eledon Pharmaceuticals or XVIVO. Federal funding was used for the preclinical trial studies leading to this work. DA is an employee of Revivicor Inc., a subsidiary of United Therapeutics, Inc. Members of the Program in Cardiac Xenotransplantation received research funding from United Therapeutics, Inc.
[1] xeno
[2] heart transplant
[3] cell free DNA
[4] proBNP
[5] noninvasive monitoring
[6] graft rejection