Aarnav Grover, United States has been granted the CareDx Travel Awards
Xenotransplantation of encapsulated human MMP-9 enhanced mesenchymal stem cells and pancreatic islets in diabetic mice
Aarnav Grover1, Anjali Verma1, Srinivasan Muthukrishnan1, Wanxing Cui2, Magali Fontaine3, Curt Civin4, Raphael P.H. Meier1.
1Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 2Cell Therapy Manufacturing Facility, Georgetown University, Washington DC, DC, United States; 3Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, United States; 4Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD, United States
Purpose: Diabetes is widespread disease, demanding lifelong insulin dependence with risks including hypoglycemia and kidney complications. Encapsulated pancreatic islet xenotransplant offers hope to broadly treat patients with diabetes, end-stage renal disease (in conjunction with a kidney xenotransplant), and hypoglycemia unawareness. However, limited engraftment of xenogeneic islets remains a barrier. Matrix Metalloproteinase-9 (MMP-9), a key molecule secreted by Mesenchymal Stem Cells (MSCs), has been shown to enhance beta cell function and islet vascularization. We tested whether encapsulated human MMP-9 enhanced MSCs and islets can facilitate and prolong diabetes in mice.
Method: MMP-9 overexpression was achieved in MSCs by two different approaches, GM-MSC1 and GM-MSC2, with 6,000-fold and 15-fold increase in MMP-9, respectively. Overexpression of MMP-9 in modified MSCs was confirmed by qPCR and ELISA. We studied the effects of MSCs and modified MSCs on pancreatic islet function and survival in vitro and in vivo. Islet Insulin secretion was evaluated in vitro by measuring glucose-stimulated insulin secretion (GSIS). Islet morphology and viability were assessed over time using FDA/PI staining. Diabetes was induced in C57BL/6 mice by intraperitoneal injection of streptozotocin (STZ) at 220 mg/kg. Diabetes was defined as glycemia levels >360 mg/dL. Encapsulated human islets were xenotransplanted intraperitoneally in diabetic mice within groups: islets alone, islets+MSCs, islets+GM-MSC1, and islets+MSC2. Blood glucose level was monitored twice a week for each mice group. Islet xenograft failure was concluded when glucose level was >360 mg/dL for three consecutive measurements.
Results: FDA/PI staining assessed islet viability post-encapsulation, indicating excellent cell viability in all the groups (Fig. A). Islets cultured with GM-MSC1 demonstrated a significantly increased insulin response compared to other groups in both co-culture and transwell settings (Fig. B). Blood sugar levels of streptozotocin-induced diabetic mice that reversed diabetes after islet transplantation are shown in Fig. C. Four mice (40%) achieved long-term graft survival in the absence of immunosuppression (i.e.>30 days). Those included one mouse transplanted with islets alone and three mice transplanted with islets+GM-MSCs (Fig. C). Overall, mice receiving islet+GM-MSC transplants showed a trend toward improved xenograft survival relative to the other groups (Fig. D). This phenomenon could result from the microenvironment provided by enhanced MMP-9 secretion, which promotes beta cell function and improves xenograft vascularization.
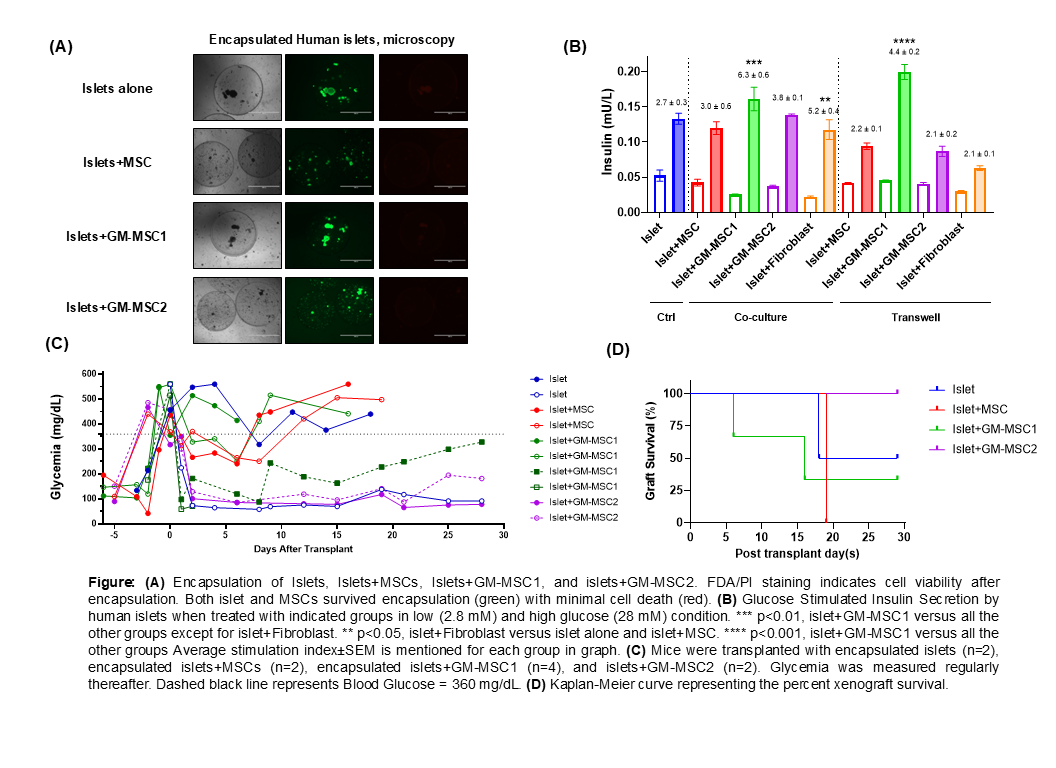
Conclusions: The present study shows that MSCs with upregulated MMP-9 expression can support microencapsulated human islets when transplanted into mice. Future investigations of MMP-9-enhanced MSCs on encapsulated islets microenvironment will help to better understand the mechanism of their favorable effect on beta cell function.
Maryland Stem Cell Research Fund Discovery Grant 2024.
[1] Human Mesenchymal Stem Cells
[2] Islet Encapsulation
[3] Xeno-Islet Survival
[4] Matrix Metalloproteinase-9