Multiplex gene-edited porcine corneal xenotransplantation in Tibetan macaques: Immune rejection mechanisms
Xiangqian Hong1, Yukun Yang2, Dengke Pan3, Xiaoming Yao1,2.
1Shenzhen Eye Hospital, Shenzhen Eye Medical Center, Southern Medical University, Shenzhen, People's Republic of China; 2Chengdu Aidi Eye Hospital, Chengdu, People's Republic of China; 3Chengdu Clonorgan Biotechnology Co. Ltd, Chengdu, People's Republic of China
Introduction: Full-thickness corneal xenotransplantation from pigs to non-human primates has used wild-type or simple transgenic donors, with few reports on grafts from more extensively gene-edited animals. We investigated immune rejection mechanisms after penetrating keratoplasty (PKP) using porcine corneas carrying triple knockout of α-Gal, Sda, and CMAH antigens (GTKO/SdaKO/CMAHKO) plus human complement-regulatory transgenes (hCD55 ± TBM) in Tibetan macaques.
Methods: Four macaques received standard PKP with gene-edited minipig grafts. Postoperatively, all animals received subconjunctival triamcinolone. Graft status was assessed by slit-lamp microscopy; rejection was defined as stromal edema > 800 µm with neovascularization. Upon clinical rejection, eyes were enucleated and examined histopathologically for inflammatory infiltrates, neovascular density, epithelial and stromal changes, and immunofluorescent markers (IgG, CD31).
Results: Early postoperative course was uneventful: graft clarity and wound integrity were maintained through day 8. By that time, two monkeys developed keratic precipitates, peripheral stromal vessels, and anterior-chamber fibrin. One graft opacified on day 19; by day 28, all grafts showed diffuse stromal edema, opacity, and neovascular ingrowth. Histology revealed dense T-lymphocyte infiltration at graft-host junctions, mixed lymphoplasmacytic and macrophagic infiltrates with abundant eosinophils, and variable angiogenesis (≥ 15 vessels/mm² in two cases). Epithelial thickness was irregular (central 45.3 ± 6.2 µm vs. peripheral 62.1 ± 8.7 µm), with posterior stromal adhesions and pigmented tissue invasion. Two macaques exhibited high microvessel density at the graft margin (28.4 ± 3.1 vessels/mm²), stromal thickness increased to 1,024 ± 89 µm, and disrupted collagen architecture. One case showed IgG+ keratic precipitates.
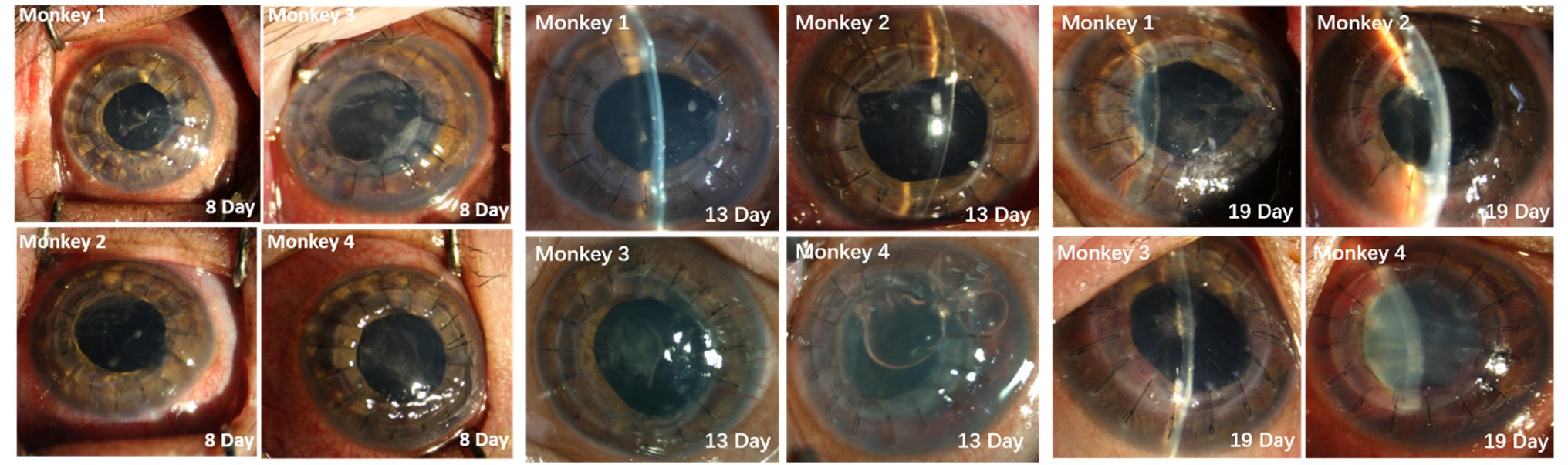
Conclusion: Gene-edited porcine corneas prevent hyperacute rejection but do not avert chronic xenogeneic rejection. Contributing factors likely include donor-recipient corneal thickness mismatch, neovascular invasion, anterior synechiae disrupting the anterior chamber-associated immune privilege deviation (ACAID), and absence of systemic immunosuppression. Histopathology suggests that mechanical mismatch at the graft edge induces neovascularization, enabling direct lymphocyte infiltration and graft attack. While extensive gene editing can reduce antigenicity, overediting may compromise donor viability and expose novel antigens. Optimizing specific combinations of gene edits—rather than maximizing edits—and leveraging the immune-privileged anterior chamber for endothelial grafts may better prolong xenograft survival and offer an alternative to allogeneic donors.
Shenzhen Science and Technology Innovation Commission, Shenzhen Science and Technology Major Program (No. KJZD20240903103305008).
[1] Graft Rejection
[2] Corneal Xenotransplantation
[3] Penetrating Keratoplasty (PKP)
[4] Multiplex Gene-Editing
[5] Pig-to-Nonhuman Primate (NHP)