Development of the replacement system for animal fetal kidneys with xenogenic nephrons: toward clinical application
Shutaro Yamamoto1,2, Shuichiro Yamanaka1, Takumi Ikeda1, Takafumi Kuroda1, Hinari Ohashi1, Nagisa Koda1, Keita Morimoto1, Kei Matsumoto1, Takahiro Kimura2, Eiji Kobayashi3, Takashi Yokoo1.
1Division of Nephrology and Hypertension, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan; 2Department of Urology, The Jikei University School of Medicine, Tokyo, Japan; 3Department of Kidney Regenerative Medicine, The Jikei University School of Medicine, Tokyo , Japan
Introduction: We are developing a renal failure therapy based on xeno-regenerative medicine, in which human iPS cell–derived nephron progenitor cells (NPCs) are combined with porcine fetal kidneys to generate chimera kidneys. To establish a chassis for chimera kidney generation, a cell-ablation system targeting NPCs is required to create a vacant nephrogenic niche. Several such systems have been developed in mouse models (1). Furthermore, using an anephric rat model, we demonstrated prolonged survival after transplantation of allogeneic fetal kidneys (2). That model demands sophisticated microsurgery techniques—creation of a integrated metanephros-bladder composite and anastomosis via a stepwise peristaltic ureter system connecting the fetal bladder to the native ureter. Translating these techniques to the mouse scale is technically challenging; thus, developing a cell-ablation system in a rat model promises to accelerate these studies.
Methods: We generated a “Six2-ATTAC9” BAC transgenic rat expressing FK506-inducible iCaspase9 under the control of the Six2 promoter. Fetal kidneys were harvested and treated with a chemical inducer of dimerization (CID) to ablate NPCs and thereby form a nephrogenic niche. These niche-prepared fetal kidneys were then repopulated in vitro by isolating and transplanting exogenous mouse NPCs, and the resulting chimera fetal kidneys were cultured on Transwell inserts.
Results: Fetal kidneys cultured on Transwell membranes with CID showed effective NPC depletion and successful formation of a nephrogenic niche. After transplanting mouse progenitor cells into these kidneys and culturing for four days, immunostaining revealed integration of donor mouse cells with rat ureteric buds and the formation of chimeric nephrons (Figure 1).
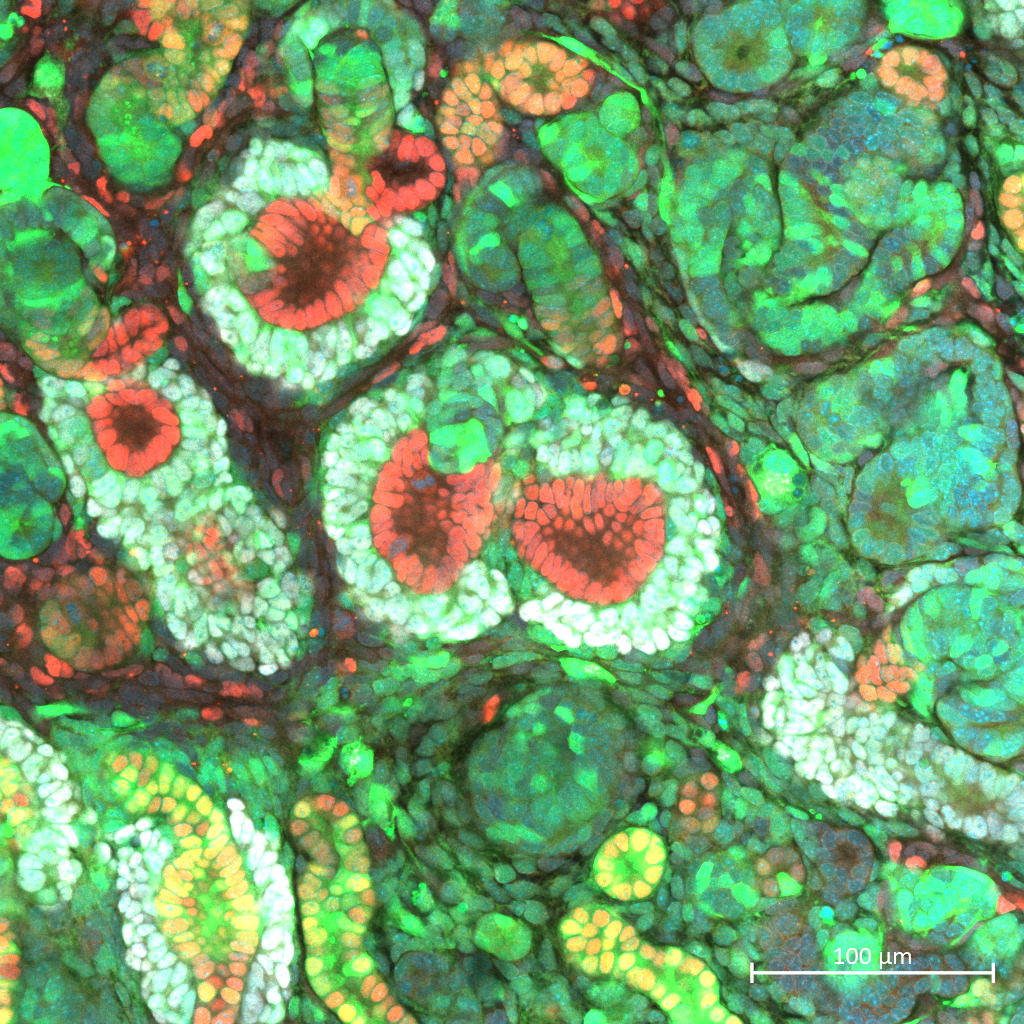
Conclusion: We have successfully up-scaled our microsurgical platform to a rat model compatible with cell ablation. This advance enables functional evaluation of chimera fetal kidneys and assessment of survival benefit in vivo, moving us closer to clinical application.
References
(1) Matsui K, Watanabe M, Yamamoto S, et al. Caspase 9–induced apoptosis enables efficient fetal cell ablation and disease modeling. Nat Commun. 2025;16:2572. doi:10.1038/s41467-025-57795-6
(2) Kinoshita Y, Kobayashi E, Matsui K, et al. Life-supporting functional kidney replacement by integration of embryonic metanephros–bladder composite tissue transplants. Kidney Int. 2025 Mar 21. doi:10.1016/j.kint.2025.02.024
T.Y. declares support from AMED [grant number JP24bm1223003] and JSPS-KAKENHI [grant number JP21K08288]. S.Yamanaka declares support from AMED [grant numbers JP22bm0704049 and JP24bm1123036], JST FOREST Program [grant number JPMJFR2011], and JSPS-KAKENHI [grant numbers JP19K17756 and JP24K11439].
[1] Fetal Kidney
[2] Replacement system
[3] Chimera Fetal Kidneys