Anjali Verma, United States has been granted the CareDx Travel Awards
Ex vivo pig liver xenoperfusion with human red blood cells and mesenchymal stem cells to enhance the endothelial barrier function and attenuate inflammation
Anjali Verma1, Kamoltip Promnares2, Aarnav Grover1, Javier Galindo1, Avneesh Singh1, Curt Civin3, Konstantin Birukov2, Muhammad Mohiuddin1, Magali Fontaine4, Raphael Meier1.
1Surgery, School of Medicine, University of Maryland, Baltimore, MD, United States; 2Anesthesiology, School of Medicine, University of Maryland, Baltimore, MD, United States; 3Pediatrics, School of Medicine, University of Maryland, Baltimore, MD, United States; 4Pathology, School of Medicine, University of Maryland, Baltimore, MD, United States
Purpose: Liver xenoperfusion with ex vivo machine perfusion (MP) has emerged as a promising strategy for the treatment of acute fulminant liver failure. MP also enables the application of donor-specific treatments, offering an opportunity to deliver immunosuppressive agents, condition the xenograft, reduce the immunogenicity before implantation. Emerging evidence suggests that MP can modulate both innate and adaptive immune responses through targeted delivery of anti-inflammatory molecules or cell-based therapies. Red blood cells (RBCs) and mesenchymal stem cells (MSCs) play key roles in reducing immunogenicity, inducing tolerance and graft endothelial barrier stabilization. Ex vivo perfusion with RBCs has been critical in preventing primary nonfunction in large animal studies and was implemented in first-in-human cases. The objective of our study is to combine RBCs and MSCs perfusion and evaluate their impact on endothelial barrier stability, and immune tolerance in the xenogeneic setting.
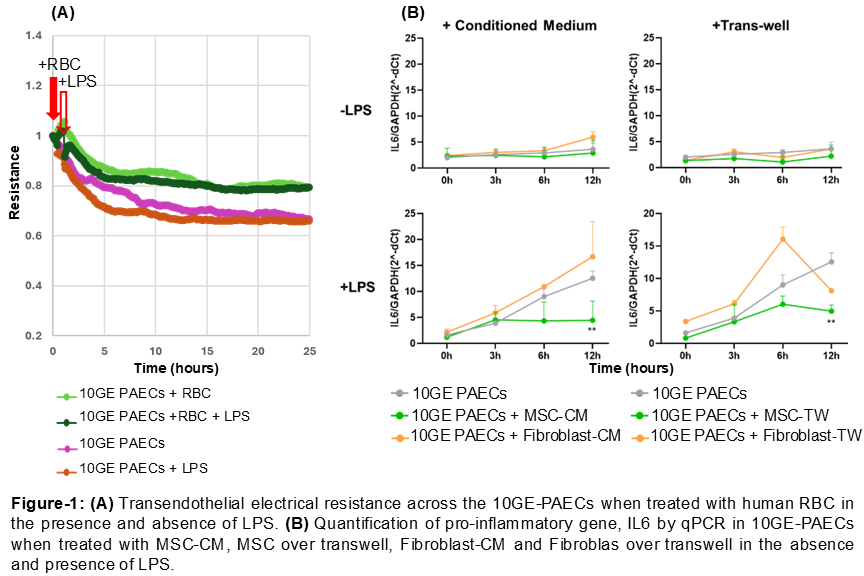
Method: We tested the effect of RBCs and MSC on 10-gene-edited (10GE) pig aortic endothelial cells (PAECs) in vitro. We measured transendothelial electrical resistance (TER) across 10GE-PAECs when treated with human RBCs using the electric cell-substrate impedance sensing system (ECIS-1600). We then treated 10GE-PAECs with human MSC-conditioned medium (MSC-CM) or human MSCs in transwell (MSC-TW). To stimulate the endothelial response, 10GE-PAECs were concomitantly treated with LPS (200 ng/ml) for 0, 3, 6, or 12 hours. qPCR was used to quantify gene expression of inflammatory cytokines, including IL-6 and IL-8.
Results: We established a system using 10GE-PAECs, where the protective effect of anti-inflammatory agents can be studied. The deleterious effects of LPS on endothelial barrier function were partially mitigated by the addition of human RBCs to 10GE-PAECs as evidenced by TER assay (Figure-1A). This suggests a potential protective role of RBCs in modulating endothelia responses to the pro-inflammatory stimuli. Furthermore, we demonstrated that both MSC-CM and MSC-TW significantly reduced IL-6 expression, a key marker of inflammation, in endothelial cells. These anti-inflammatory effects were consistent both under basal conditions and upon LPS-induced inflammatory challenge (Figure-1B), highlighting the robust immunomodulatory potential of MSC secretome. In contrast, fibroblast cells and their conditioned medium (CM), used as controls, did not mitigate endothelial cell inflammation (Figure-1B).
Conclusions: These data suggest that RBCs have the potential to stabilize the endothelial barrier and mitigate inflammation. Additionally, MSCs reduce endothelial activation and inflammation, which is critical for preventing the initial cytokine storm that provokes accelerated rejection and a pro-thrombotic environment in xenotransplantation.
[1] Xenoperfusion
[2] Human Red Blood Cells
[3] Human Mesenchymal Stem Cells
[4] Immune tolerance