Anti-pig antibodies in swine veterinarian serum: Implications for clinical xenotransplantation
Guerard Byrne1,2, Christopher GA McGregor1,2.
1Department of Surgery, University of Minnesota, Minneapolis, MN, United States; 2Institute of Cardiovascular Sciences, University College London, London, United Kingdom
Introduction: Clinical cardiac and renal xenotransplantation and human decedent studies clearly demonstrate that hyperacute rejection of genetically engineered porcine organs, devoid of the major xenogeneic glycans, can reliably be avoided but that antibody mediated rejection (AMR) continues to limit graft survival. The clinical immune response to triple knockout (TKO) donor pigs is poorly understood but under these conditions AMR may be directed to pig endothelial cells protein antigens including, but not restricted to, SLA antigens.
Methods: We previously identified porcine glycans and proteins which are immunogenic after cardiac xenotransplantation in non-human primates. In this study we use fluorescence barcoded human embryonic kidney cells (HEK) and HEK cell lines expressing porcine glycans (Gal and SDa) or proteins (tetraspanin-29 [CD9], membrane cofactor protein [CD46], protectin, membrane attack complex inhibition factor [CD59], endothelial cell protein C receptor, and Annexin A2) to screen antibody reactivity in human serum (Figure 1A and B). Anti-pig reactivity in serum from 160 swine veterinarians was analyzed as a serum source with potential occupational immune challenge from porcine tissues and pathogens.
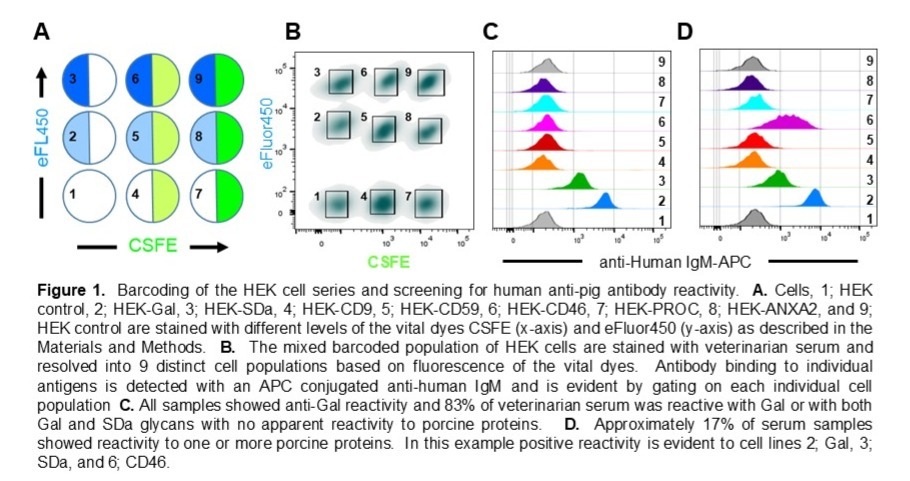
Results: Human IgM binding was evident to Gal or Gal and SDa glycans in 84% of serum samples, with no apparent binding to pig proteins (Figure 1C and Figure 2A and B). IgM binding to porcine proteins, primarily CD9 and CD46, previously identified as immunogenic in pig to nonhuman primate cardiac xenograft recipients, was detected in 28 of the 160 swine veterinarian samples (16%). The level of antibody binding to pig proteins was comparable to anti-SDa reactivity and often exceeded 5 standard deviations above background binding to control HEK cells (Figure 1D and 2C).
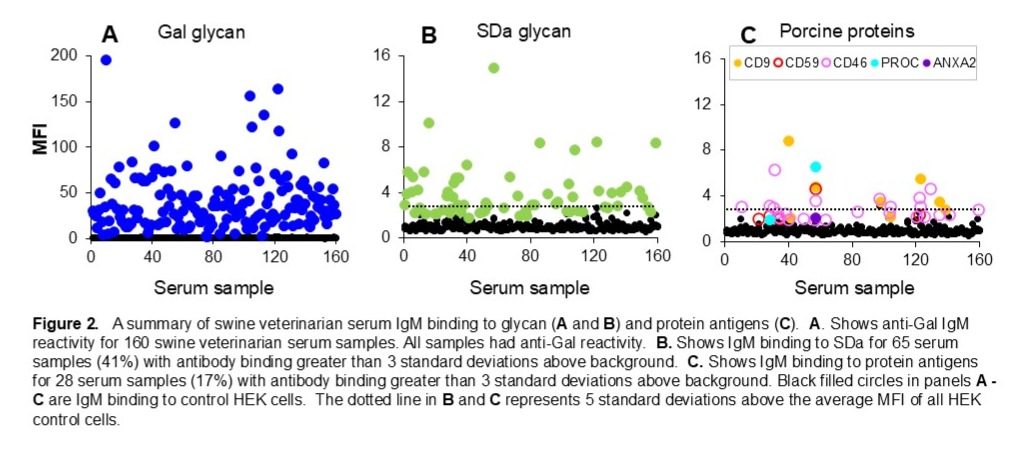
Conclusions: These results suggest that the human non-Gal immune response to clinical xenotransplantation will not be restricted to swine SLA but may extend to a range of porcine endothelial cell membrane proteins. Sera from clinical xenotransplant recipients should be comprehensively analyzed to define a panel of commonly immunogenic porcine antigens. Displaying such an array of porcine non-Gal protein antigens on cells or at high density on solid substrates will create novel and highly sensitive assays to support clinical xenotransplantation by screening clinical blood products, monitoring patient immune responses to detect AMR and assessing the effectiveness of therapies to reverse AMR.