Development of a humanized mouse model to assess neonatal porcine vs human islet engraftment and rejection
Purushothaman Kuppan1,2, Joy Paramor1,2, Mandy Rosko1,2, Chelsea Castro1,2, Nerea Cuesta-Gomez1,2, Arghyadip Bose1,2, Riley Reid1,2, Karen Seeberger1,2, Andrew R. Pepper*1,2, Gregory S. Korbutt*1,2.
1Alberta Diabetes Institute, University of Alberta, Edmonton, AB, Canada; 2Department of Surgery, University of Alberta, Edmonton, AB, Canada
Dr. Gregory S. Korbutt. Dr. Andrew R. Pepper.
Introduction: T-cell-mediated immune rejection remains a significant translational hurdle in clinical islet transplantation. A comprehensive understanding of how the adaptive immune system responds to an islet graft is essential for developing strategies to prevent islet rejection. Establishing a human immune system in mice through reconstitution with human peripheral blood mononuclear cells (hPBMCs) can provide a valuable model for elucidating the adaptive immune responses to allogeneic and xenogeneic islet grafts. The objective of this study was to assess islet rejection in humanized NSG MHC I/II double knockout (NSG) mice transplanted with either human (HIs) or neonatal porcine islets (NPIs).
Methods: Diabetic NSG mice were transplanted with either 1500 HI (n=5) or 3000 NPIs (n=12) under the kidney capsule. Graft function was assessed by weekly non-fasting blood glucose (BG) measurements. After animals achieved normoglycaemia, they received 40x106 hPBMCs intraperitoneally, and islet graft rejection was subsequently assessed by measuring non-fasting BG. Both rejected and non-rejected grafts were collected for histological analysis.
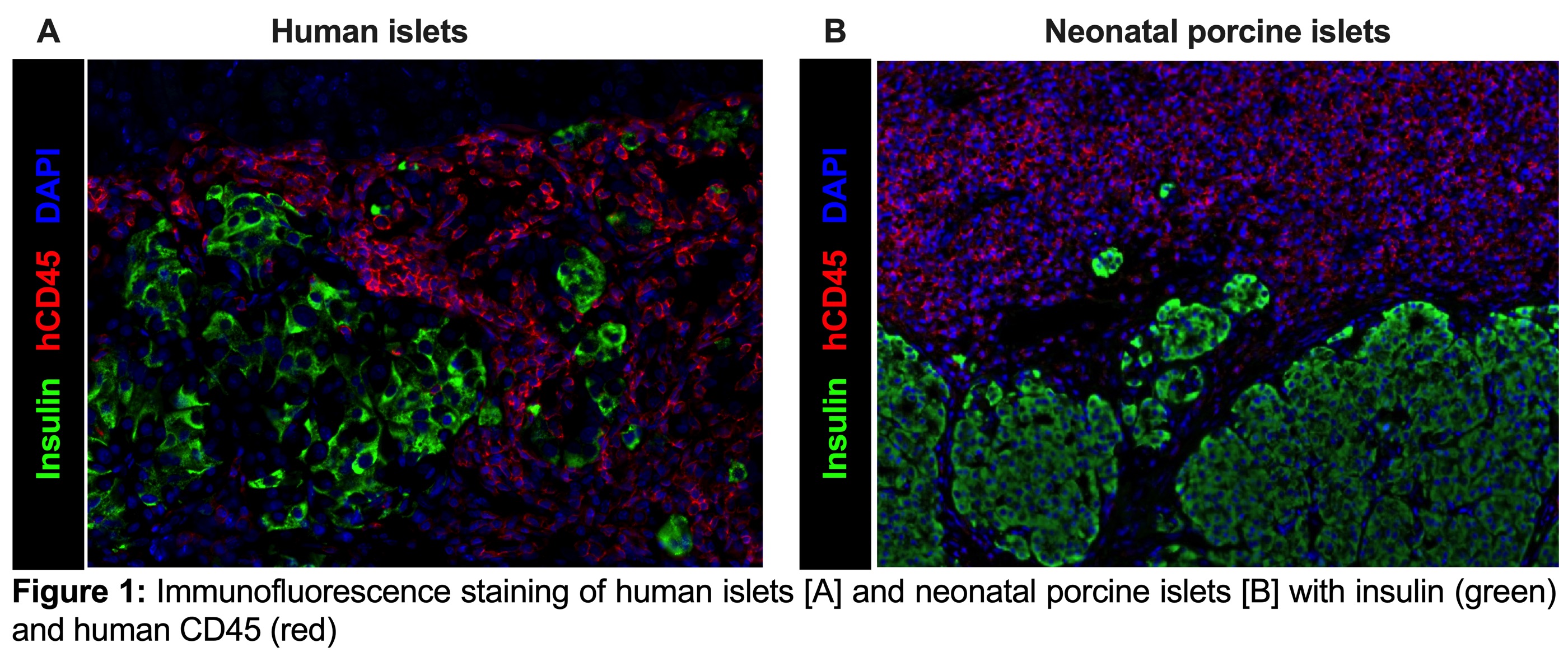
Results: All HI recipients (5/5) became normoglycemic within 2 days post-transplant and maintained normoglycemia for 40 days until administration of hPBMCs. After hPBMC infusion, 100% of the animals reverted to a hyperglycemic state within 11.2±1.7 days. Of the 12 NSG mice transplanted with NPIs, 10 mice achieved normoglycemia (10/12: 83%) within 10 to 27 weeks post-transplant and were administered hPBMCs when normoglycemia was achieved. Following hPBMCs reconstitution only 10% of NPI recipients (1/10), became hyperglycemic. Survival graft-bearing nephrectomy was conducted on normoglycemic recipients, and the subsequent return to hyperglycemia confirmed graft dependent function. Immunofluorescence staining confirmed rejection of HIs as shown by significant hCD45 infiltration and few surviving ß-cells [Figure 1A]. Similar morphology was observed in the one NPI transplanted mouse that demonstrated rejection. In contrast, NPI grafts in mice that did not reject exhibited extensive hCD45 infiltration and no reduction in ß-cell mass [Figure 1B].
Conclusion: These data demonstrate that human islets are highly susceptible to hCD45 cell infiltration and subsequent rejection in a humanized mouse model. In contrast, NPI grafts become infiltrated with hCD45+ cells however with limited or no islet destruction, thereby suggesting that NPIs could be potentially hypoimmunogenic.
[1] Humanized mouse model
[2] Neonatal porcine islets
[3] Islet xenotransplantation
[4] Human peripheral blood mononuclear cells
[5] Human islets