Engineered MSCs releasing CCL22 remodel the local immune microenvironment to promote graft tolerance
Yanan Li1, Wei Wang1, Xiaoqian Ma1, Juan Zhang1, Lu Cao1, Xingshi Gu1.
1Cell Transplantation and Gene Therapy Institute, The Third Xiangya Hospital, Central South University, Changsha, People's Republic of China
Introduction: Transplantation is essential for patients with severe organ damage or end-stage organ failure. However, challenges like immune rejection, chronic inflammation, infections, and poor graft survival severely limit long-term transplant success. While immunosuppressive therapies prevent rejection, their long-term use often leads to serious side effects. Thus, inducing local immune tolerance without extensive immunosuppression remains crucial. Chemokine CCL22 plays roles in various physiological and pathological processes through chemotactic and non-chemotactic mechanisms. This study explores engineered mesenchymal stem cells (MSCs) that locally release CCL22, examining their ability to reshape the immunosuppressive microenvironment.
Methods: We established a murine allogeneic skin transplantation model, performing graft rejection scoring across different experimental groups. Grafts were analyzed using H&E staining, immunohistochemistry, immunofluorescence, and ELISA. In vitro, the function of CCL22 was assessed through mixed lymphocyte reaction (MLR) assays, flow cytometry, and ELISA. Mechanistic insights were obtained by blocking the CCR4 receptor. Additionally, MSCs overexpressing CCL22 (CCL22-OE MSCs) were generated and subcutaneously transplanted into mice, with subsequent analyses of the transplantation sites.
Results: CCL22 markedly prolonged graft survival, reduced inflammation, enhanced regulatory T cell (Treg) infiltration, and lowered rejection scores in the mouse model. In vitro assays showed CCL22 efficiently recruited Tregs. MLR assays demonstrated that CCL22 increased Tregs' suppressive capabilities against T-cell proliferation. Flow cytometry revealed CCL22 upregulated Treg-specific transcription factor FOXP3 and surface markers GITR and ICOS. ELISA indicated that CCL22 treatment elevated production of immunoregulatory cytokines IL-10 and TGF-β, alongside promoting Ca²⁺ influx. Mechanistically, these effects depended on the CCR4 receptor, as demonstrated by the loss of Treg recruitment and function upon administration of CCR4 antagonist C-021. Immunofluorescence analyses showed that subcutaneous transplantation of CCL22-OE MSCs significantly enhanced local Treg recruitment, markedly decreased M1 macrophages, and increased M2 macrophages. Additionally, in vitro co-culture experiments revealed that CCL22-OE MSCs induced macrophage polarization toward the M2 phenotype.
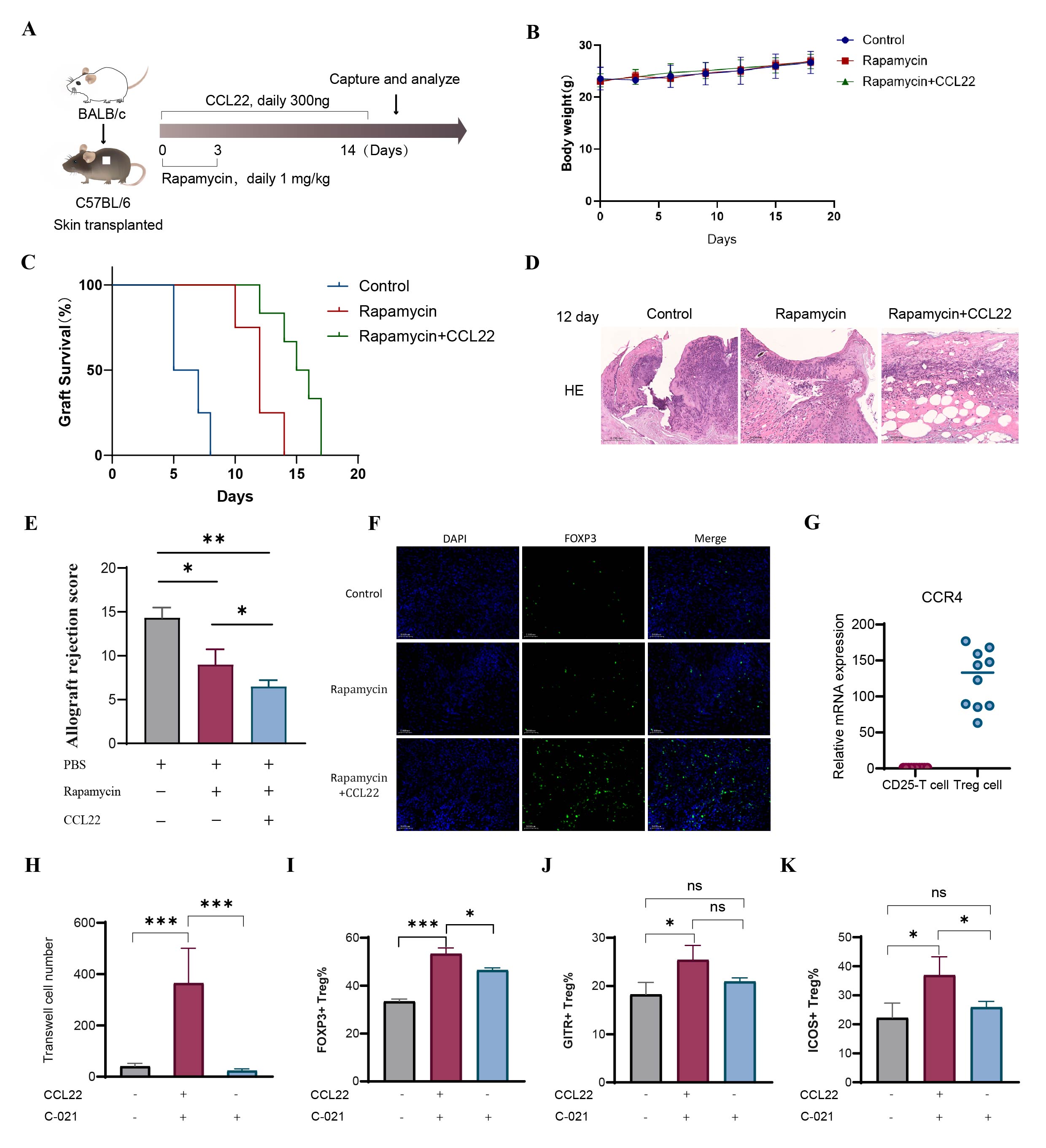
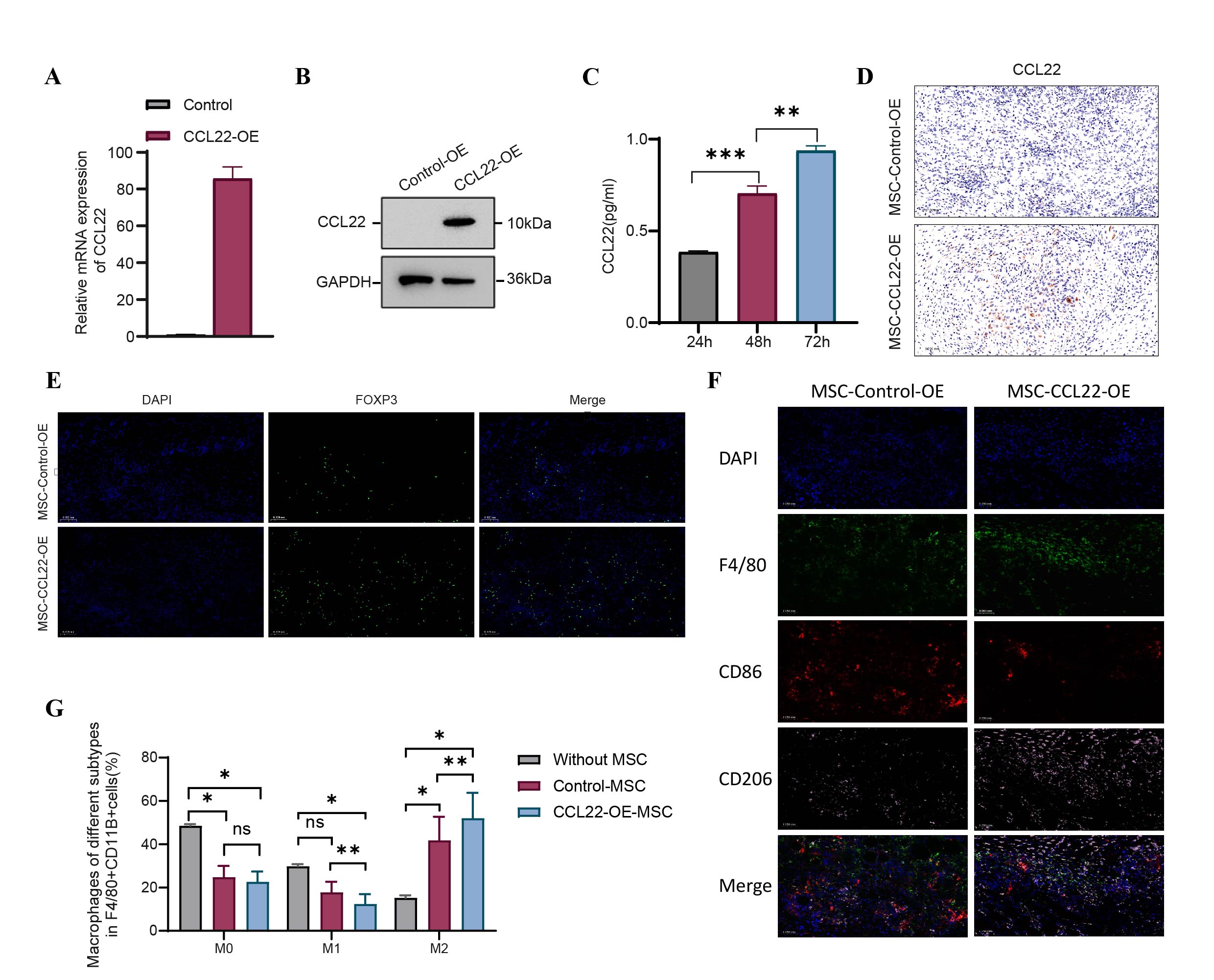
Conclusion: Engineered MSCs secreting CCL22 robustly recruited immunosuppressive Tregs and strengthened their suppressive function via the CCL22-CCR4 pathway. Concurrently, these cells promoted local macrophage polarization to the anti-inflammatory M2 phenotype, increased local anti-inflammatory cytokine secretion, reshaped the immune microenvironment, suppressed graft rejection, and significantly prolonged graft survival. This study enhances our understanding of chemokines in transplantation, providing promising insights to reduce immune rejection and graft loss.
The Key proiect of the Science and Technology Program of Hunan Province (Grant No.2023ZJ1100) . The National Narural Science Foundation of China (Grant No:82372071 and 82272102).
[1] Graft tolerance
[2] Immune microenvironment remodeling
[3] CCL22
[4] Engineered MSCs