Dr. Sykes is the Michael J. Friedlander Professor of Medicine and Professor of Microbiology & Immunology and Surgical Sciences (in Surgery), Columbia University. Dr. Sykes is the founding Director of the Columbia Center for Translational Immunology and serves as Director of Research for the Transplant Initiative and as Director of Bone Marrow Transplantation Research at Columbia. Dr. Sykes joined Columbia University in April, 2010 from Massachusetts General Hospital/Harvard Medical School, where she was the Harold and Ellen Danser Professor of Surgery and Professor of Medicine (Immunology) and Associate Director of the Transplantation Biology Research Center. Dr. Sykes has over 40 years’ experience in transplantation biology and Type 1 diabetes research, including translational research from animals to clinical trials and mechanistic studies of human transplant recipients. She is Past President of the Federation of Clinical Immunology Societies (FOCIS) and of the International Xenotransplantation Association. Dr. Sykes received numerous honors and awards, including the Medawar Prize in 2018, membership in the National Academy of Medicine and the Association of American Physicians. She was awarded the Barry Prize by the American Academy of Sciences and Letters in 2024 and the 2025 Thomas E. Starzl Prize in Surgery & Immunology.
Evidence for human thymopoiesis in a porcine thymus graft and T cell tolerance to the source pig in a living human recipient of a porcine thymokidney xenotransplant
Farshid Fathi1, Pinar Gur Cetinkaya1, Yasmeen S Saad1, Benjamin Vermette1, Jeffrey Stern2, Karen Khalil2, Jacqueline Kim2, Ian Jaffe2, Imad Aljabban2, Lars Burdorf3, Adam D Griesemer2, Robert A Montgomery2, Megan Sykes1,4,5.
1Columbia Center for Translational Immunology, Department of Medicine, Columbia University Medical Center, New York, NY, United States; 22Department of Surgery, Transplant Institute, New York University Langone Health, New York, NY, United States; 3United Therapeutics Corporation, Research Triangle Park, New York, NY, United States; 4Department of Surgery, Columbia University Medical Center, , New York, NY, United States; 5Department of Microbiology and Immunology, Columbia University Medical Center, New York, NY, United States
Study’s purpose: Porcine thymic transplantation tolerizes xenogeneic recipients to the porcine source animal in animal models. A 54-year-old woman with complex comorbidities received a composite thymokidney transplant from an GGTA1 knockout pig, achieving initial renal function before xenograft failure for non-immunological reasons on postoperative day (POD) 47.
Methods: Serial peripheral blood mononuclear cell (PBMC) samples were analyzed by spectral FCM, and mixed lymphocyte reactions (MLRs) were performed on purified T cells to assess tolerance. Thymocytes were isolated from the thymic portion of the xenograft explant and analyzed using spectral FCM.
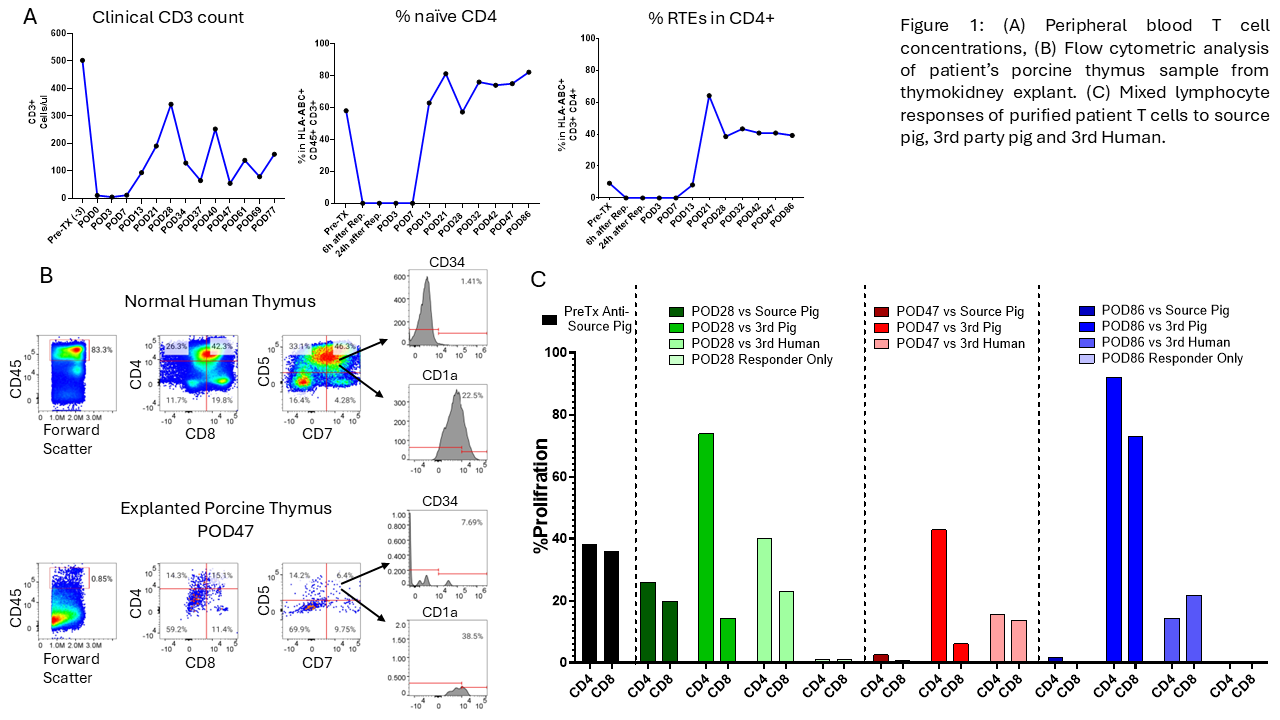
Results: T cell levels in peripheral blood declined to 10/µL post-induction therapy and recovered to a peak of 342/µL by POD28. By POD21, 64% of the recovering CD4 T cells displayed a CD45RA+CCR7+ "naïve" phenotype and expressed CD31, consistent with recent thymic emigrants (RTEs). Pre-transplant CD4 T cells included <10% RTEs (Figure 1A). FCM analysis on cells from the POD47 thymic xenograft revealed human CD45+ leukocytes (0.85%), including double-positive (CD4+CD8+), single-positive, and double-negative thymocytes. The double-negative cells expressed markers such as CD5, CD7, CD1a, and CD34, consistent with human T cell progenitors in the porcine thymus (Figure 1B). MLR results demonstrated progressive hyporesponsiveness toward the source pig with preserved responses to third-party pig and allogeneic human cells, achieving full donor-specific unresponsiveness by POD47 and POD86 (Figure 1C).
Conclusion: These findings suggest that a porcine thymus in a thymokidney xenograft supported human thymopoiesis and the development of T cells tolerant to the pig. Thymic transplantation is a promising approach to achieving T cell tolerance in xenotransplant recipients.