Early xeno-derived cell-free DNA elevation as a noninvasive biomarker of kidney xenograft rejection in pig-to-nonhuman primate xenotransplantation
Joon Young Jang1, Il Hee Yun1, Kyu Hyun Han1, Beom Seok Kim1, Ik Jin Yun2, JAESEOK YANG1.
1Internal medicine, Yonsei University Severance Hospital, SEOUL, Korea; 2Surgery, Konkuk University School of Medicine, SEOUL, Korea
Introduction: Kidney xenograft biopsy is a standard diagnostic method of xenograft rejection. Recently, donor-derived cell-free DNA (cfDNA) has emerged as a promising early, non-invasive biomarker for allograft rejection; however. its utility as a screening test for xenograft rejection remains unclear. This study investigates whether xeno-derived cfDNA levels can predict xenograft rejection and have an association with severity of histological and immunological injury in kidney xenograft tissues.
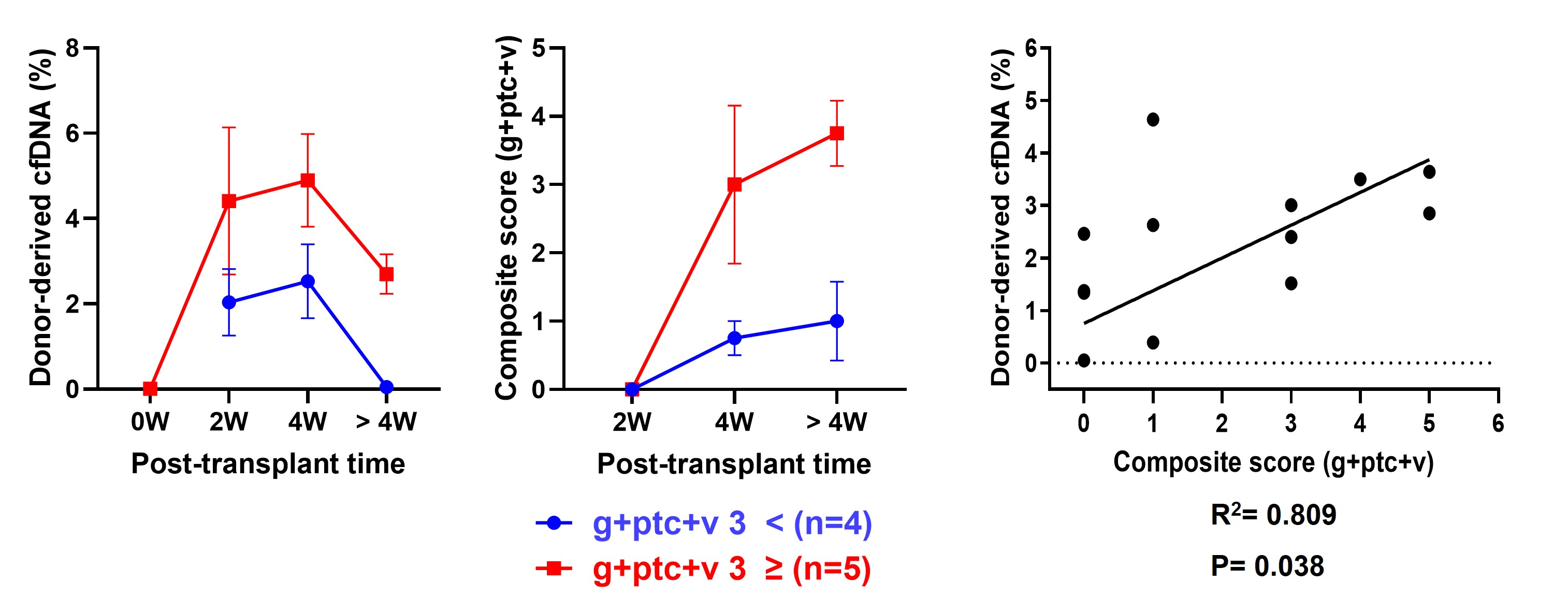
Methods: Kidneys from multiple genetically-engineered pigs (knock-out for GGTA1, CMAH, iGb3s, or B4GalNT2; knock-in for human CD39, CD46, CD55, or thrombomodulin) were transplanted into cynomolgus monkeys under a few immunosuppressive regimens, which consisted of thymoglobulin, rituximab, anti-CD154, abatacept or anti-ICAM-1 (MD-3), anti-C5 inhibitor (crovalimab), and triple maintenance immunosuppressants including prednisolone, tacrolimus, and mycophenolate mofetil. Kidney xenograft biopsies at 2, 4 weeks, and later timepoints were diagnosed based on Banff pathologic classification 2022 including transcriptomic analysis and immunohistochemical staining (CD3, CD68. IgG, C4d, membrane attack complex [MAC], CD61). Recipients were categorized into two groups according to a composite score of g + ptc + v at xenograft biopsy: mild (score <3) vs. severe (score ≥3) injury groups. Pig-derived cfDNA was serially measured using a random next generation sequencing technology at pre-transplant timepoint and post-transplant 2 week, 4 week, and later timepoints.
Results: Among nine recipients, 8 monkeys experienced antibody-mediated rejection. Four monkeys belonged to the mild injury group, while the remaining five belonged to the severe injury group at 4 weeks post-transplantation. Overall, cfDNA levels were already elevated at post-transplant 2 weeks, when the composite scores of xenograft biopsies were still low. cfDNA levels remained high at 4 weeks, when the composite scores increased. Then, cfDNA levels decreased beyond 4 weeks posttransplantation, when the composite scores were still elevated. These data suggest that cfDNA levels precede changes of the composite scores in xenografts, highlighting its potential as an early biomarker of rejection. Furthermore, cfDNA levels in the severe-injury group (mean, 4.89%) showed a higher tendency than those in the mild-injury group (mean, 2.53%), while cfDNA level in case of no-rejection was only 0.39%. There was a strong correlation between cfDNA levels and the composite scores (R2=0.809, P=0.038). In parallel, immunohistochemical staining showed that cellular infiltration (CD3+, CD68, CD61+) and deposition of MAC and IgG deposition were markedly elevated in the severe-injury group.
Conclusion: Serial levels of xeno-derived cfDNA are promising as an early, noninvasive biomarker of xenograft rejection, which could be confirmed by subsequent xenograft biopsy.
[1] Graft Rejection
[2] Diagnosis
[3] Monkey
[4] Pig
[5] Kidney