
Real-time intraoperative quantification of xenograft blood flow into a porcine xenothymokidney in a living human with a left ventricular assist device
Jacqueline Kim1, Imad Aljabban1, Aprajita Mattoo1, Vasishta Tatapudi1, Edward Y. Skolnik2, ian S. Jaffe1, Karen S. Khalil1, Molly S. Ratner1, Elaina P. Weldon1, David Ayares3, Bernard S. Kadosh4, Randal I. Goldberg4, Tajinderpal Saraon4, Alex Reyentovich4, Deane E. Smith5, Jeffrey M. Stern1, Adam Griesemer1, Robert A. Montgomery1, Nader Moazami5.
1New York University Langone Transplant Institute, New York, NY, United States; 2Department of Medicine, New York University Langone Health, New York, NY, United States; 3Revivicor, Inc., Blacksburg, VA, United States; 4Department of Cardiology, New York University Langone Health, New York, NY, United States; 5Department of Cardiothoracic Surgery, New York University Langone Health, New York, NY, United States
Introduction: Patients with concomitant end-stage heart and renal disease are not considered for left ventricular assist device (LVAD) implantation as destination therapy due to high mortality rates. Therefore, the fluid dynamics of blood flow through a newly transplanted renal graft, let alone a porcine xenograft, in the setting of a LVAD have not been investigated. In this study, we quantify intra-operative real-time xenograft blood flow through a newly transplanted xenothymokidney renal artery in a living recipient with an LVAD.
Methods: One week after implantation of a continuous flow mechanical circulatory device (Heartmate 3 LVAD), a 200.0-gram alpha-Gal knockout xenothymokidney was transplanted into a 170-cm female living human recipient. Intra-operatively, transit time flow measurement was utilized to quantify real-time volume flow through the newly transplanted porcine renal artery. LVAD pump speed was set to 4800 rpm peri-operatively and systemic hemodynamics measured continuously during the xenotransplantation. Approval for this procedure was obtained from the FDA under a single patient expanded access Investigational New Drug application and the New York University Grossman School of Medicine Institutional Review Board.
Results: Baseline blood flow through the recipient’s proximal left external iliac artery (L EIA) just prior to xenograft implantation was 240 mL/min, with a systemic mean arterial pressure (MAP) of 76 mmHg and an estimated systemic flow of 3.4 L/min. Immediately after xenograft reperfusion, proximal L EIA blood flow increased to 500 mL/min (2.1-fold), with 160 mL/min xenograft renal artery blood flow (4.7% of systemic blood flow) and 226 mL/min distal L EIA blood flow. 30 minutes after xenograft reperfusion, with systemic MAP 73 mmHg and estimated systemic flow of 3.6 L/min, proximal L EIA blood flow further increased to 694 mL/min (2.9-fold) and xenograft renal artery blood flow increased to 454 mL/min (2.8-fold from reperfusion, 12.6% of systemic flow). Distal L EIA flow remained grossly unchanged at 230 mL/min.
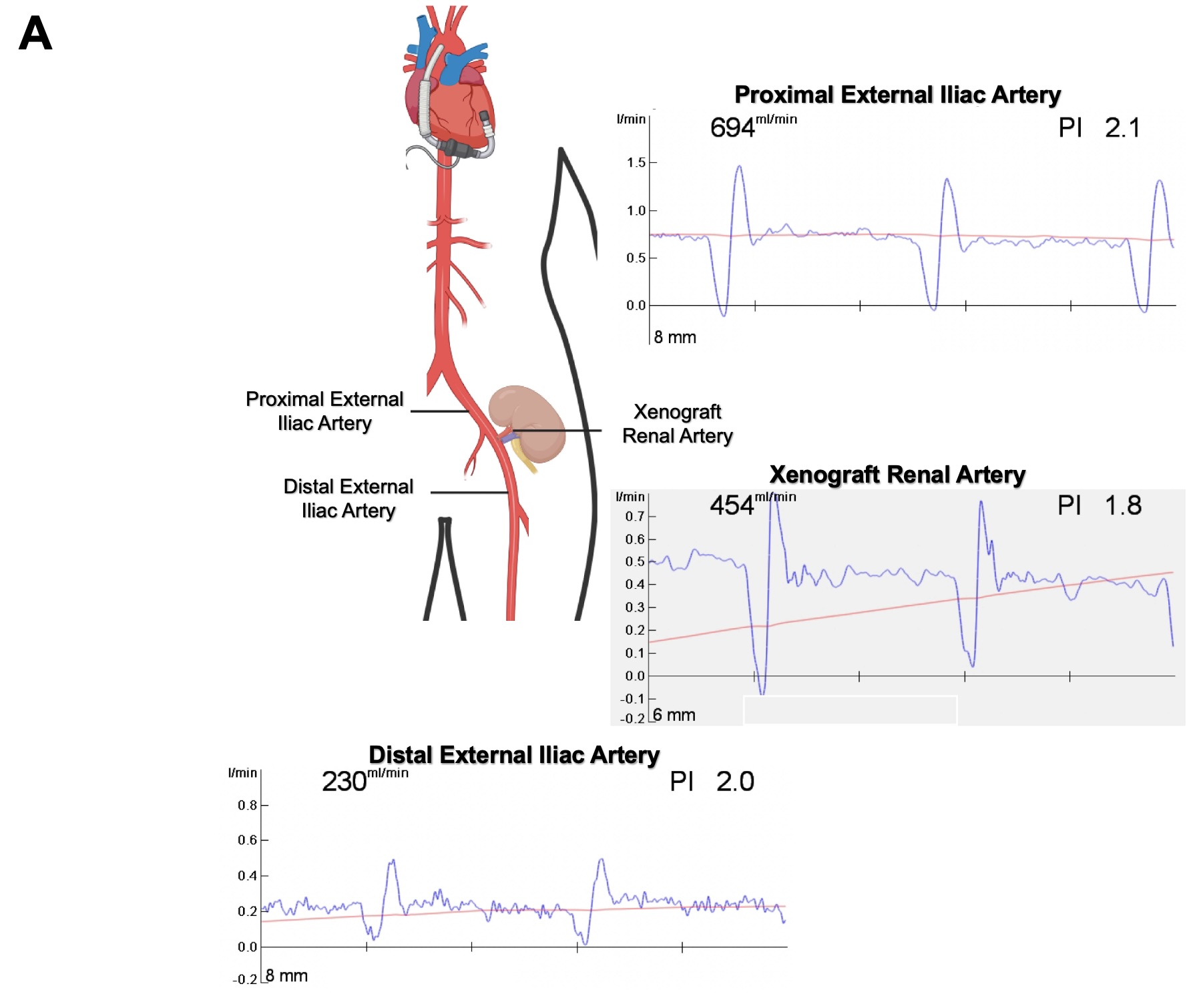
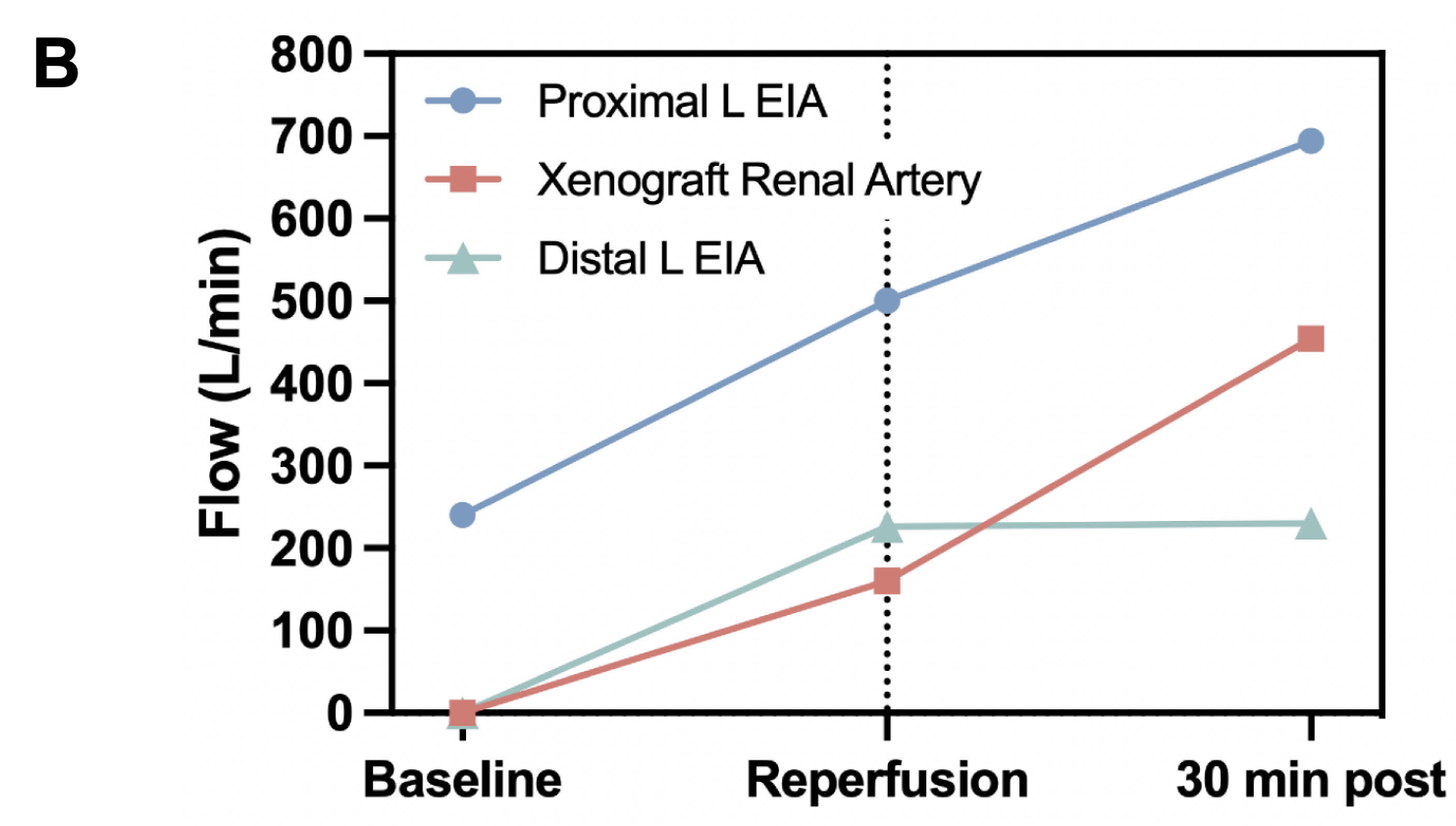
Discussion: In this single-patient study of intraoperative xenograft kidney blood flow dynamics, we observed rapid adaptations in real-time blood flow through a newly transplanted xenokidney renal artery, suggesting that the porcine xenograft exhibits brisk compensatory physiologic adaptations in a human recipient even with an LVAD.
Research funding: United Therapeutics, PBC.
[1] renal xenograft
[2] end-stage heart failure
[3] end-stage renal disease
[4] mechanical circulatory device