Sarah L Leventhal, United States has been granted the Swiss National Science Foundation Scientific Congress Awards
Clinical Correlations with Longitudinal Echocardiography and Left Ventricular Global Longitudinal Strain in the First and Second Genetically Modified Porcine to Human Cardiac Xenotransplantations
Sarah Leventhal1, Muhammad Mohiuddin3, Javier Galindo3, Andrew Tully3, Sarah Cipriano1, Marco Oldsman1, Alison Grazioli2, Timm Dickfeld1, Allison Lankford4, Akash Shah3, Brian Barr1, Sadegh Asadi1, Albert J Hicks1, Anuj Gupta1, Avneesh Singh3, Erika Feller5, David Ayares6, Bartley P Griffith3, Susie N Hong-Zohlman1, Manjula Ananthram1.
1Cardiology, Internal Medicine, University of Maryland School of Medicine, Baltimore, MD, United States; 2Internal Medicine, University of Maryland School of Medicine, Baltimore, MD, United States; 3Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 4Obstetrics, Gynecology and Reproductive Sciences, University of Maryland School of Medicine, Baltimore, MD, United States; 5Cardiology, Medstar Health, Baltimore, MD, United States; 6Revivicor, United Therapeutics, Blacksburg, VA, United States
Introduction: After heart transplant, left ventricular global longitudinal strain (LV-GLS) is often used with invasive testing such as right heart catheterization to better understand graft health. Cardiac power output-to-mass (CPOM) is a valuable noninvasive prognosticator in patients with LV dysfunction, with higher CPOM correlating with more mechanically efficient ventricles. Our institution performed the first two genetically modified porcine cardiac xenotransplantations in humans. Serial longitudinal transthoracic echocardiograms (TTE) with LV-GLS were performed for both patients to identify evidence of xenograft dysfunction. For both recipients, marked myocardial hypertrophy with worsening LV-GLS were noted at the time of graft failure. It was hypothesized that a ratio of posterior papillary muscle thickness (PPMT) to end diastolic diameter (EDD) would correlate with LV-GLS in this specific clinical situation. It was also hypothesized that cardiac power output (CPO) and CPOM would correlate with LV-GLS, and therefore predict xenotransplant graft failure and mortality.
Methods: Serial TTE measurements obtained included LV-GLS, PPMT, EDD, and parameters for calculation of LV mass by Devereux formula and CPOM (LVOT VTI, LVOT diameter, LV internal diameter end diastole (LVIDd), interventricular septum thickness (IVSd), and left ventricular posterior wall thickness (PWd)). Peason’s correlation co-efficients between LV-GLS and echocardiographically-derived CPO and CPOM (CO = LVOT area x LVOT VTI x HR; CPO = CO x MAP /451; LVM = 0.8(1.04((LVIDd + IVSd +PWd)3 - LVIDd3)) + 0.6; CPOM = (CPO x 100)/LVM) were calculated. Serial echocardiographic measurements and ratios were subsequently compared to endomyocardial biopsies and clinical correlation, including graft failure and death.
Results: For both xenografts, the LV-GLS worsened by an average of 42.3% (CI 42.2 - 42.4%) when the PPMT/EDD ratio reached between 0.5 and 0.6, demonstrating a strong positive linear correlation (r=0.81, p<0.001), at which point the xenografts terminally failed due to diastolic and/or systolic dysfunction. For the second xenograft, there was a strong negative correlation between CPO and LV-GLS, both with contrast (r=-0.80, p<0.001) and without contrast (r=-0.96, p<0.01). There was also a strong negative correlation for the second xenograft between LV-GLS without contrast and CPOM (r=-0.96, p=0.01).
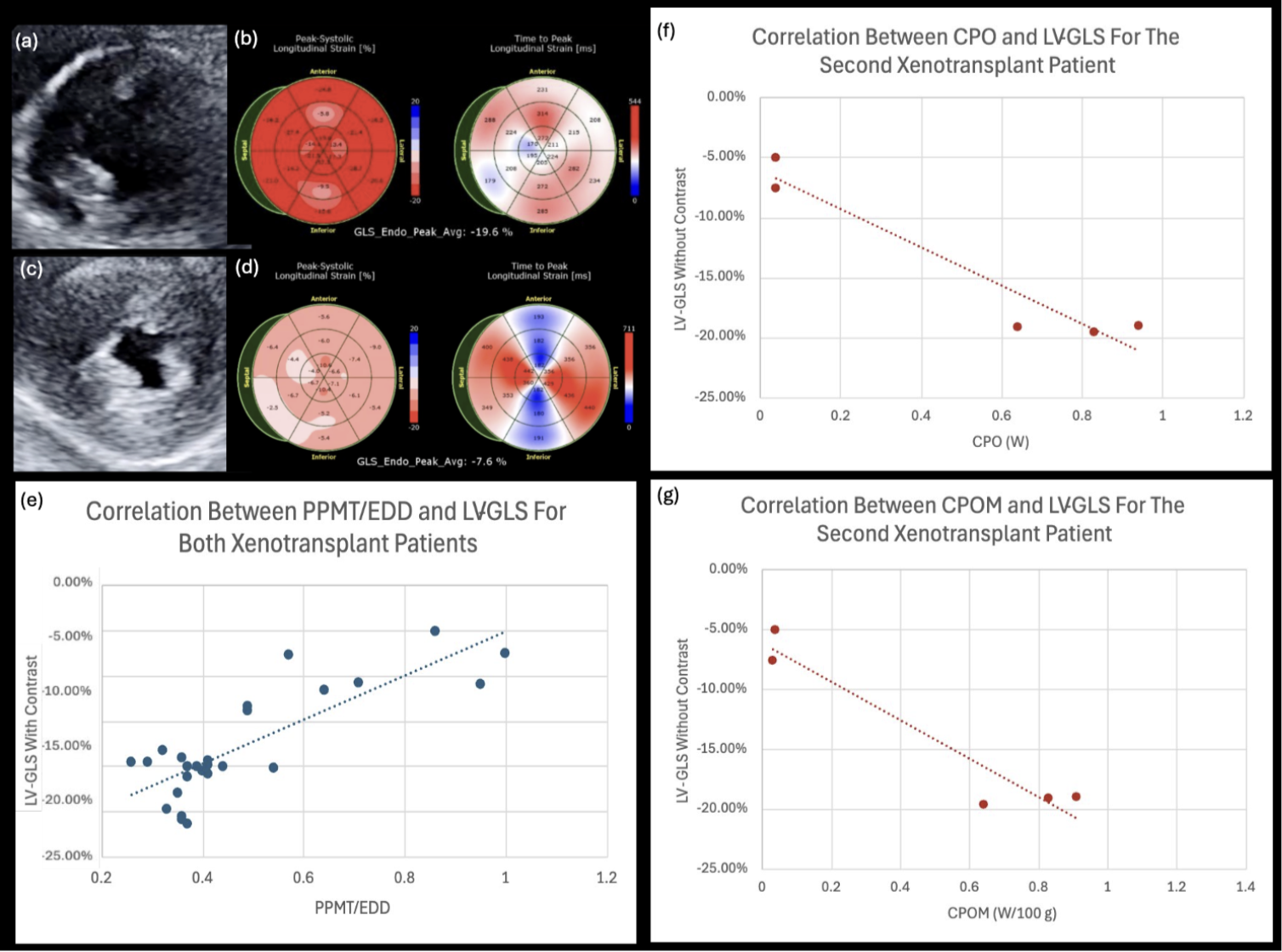
Conclusion: Increased ratio of PPMT to EDD, decreased CPO, and decreased CPOM were associated with worsening LV-GLS, cardiac xenotransplant graft failure, and recipient mortality. TTE with LV-GLS provided real-time clinical and noninvasive histological correlation in the first and second cardiac xenotransplants. Such serial noninvasive measurements identifying early graft failure will be crucial for future monitoring and management after xenotransplantation.
Revivicor, A United Therapeutics Company.
[1] Cardiac xenotransplantation
[2] Left ventricular global longitudinal strain
[3] Cardiac power output
[4] Cardiac power output-to-mass
[5] Transthoracic echocardiography