Michael A. Cole, United States has been granted the CareDx Travel Awards
Complement monitoring in cardiac xenotransplantation: Insights from the second porcine-to-human live heart transplant
Michael Cole2, Andy Tully1, Alison Grazioli1, Javier Galindo1, Avneesh K Singh1, Idris Boudhabhay4, Erwan Morgand3, Fariza Mezine3, Lubka Roumenina4, Alexandre Loupy3, Robert Brodsky2, David Ayares5, Bartley P Griffith1, Muhammad M Mohiuddin1.
1Cardiothoracic Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 2Department of Medicine, Division of Hematology, Johns Hopkins University, Baltimore, MD, United States; 3 Paris Institute for Transplantation and Organ Regeneration, INSERM U970, Université Paris Cité, Paris, France; 4InC2 lab: Inflammation, complement and cancer, INSERM UMRS 1138 , Centre de Recherche des Cordeliers, Paris, France; 5Revivicor Inc., Blacksburg, VA, United States
Background: Activation of complement is a major barrier in xenotransplantation. We report a detailed monitoring strategy in a 58-year-old man who received a 10-gene–edited porcine heart. Despite expression of human complement regulators CD46 (membrane cofactor protein) and CD55 (decay-accelerating factor) in the xenoheart, early biopsies revealed significant complement deposition, prompting changes in therapeutic approach.
Methods: The initial strategy for the inhibition of complement used the C1 esterase inhibitor (C1INH) to target classical/lectin pathways. After the first endocardial biopsy performed on day 13, the endomyocardial biopsy (EMB) showed diffuse capillary C3d and C4d deposition, therapy was switched to a C5 inhibitor, eculizumab. We monitored complement activity via immunohistochemical staining (C3c, C3d, C4d, C5b-9) on biopsies, serum soluble C5b-9 (sMAC) levels via ELISA, and a bioluminescent modified Ham test (bmHam) using HEK293 cells lacking complement regulators (CD46, CD55, CD59). The bmHam assay measured serum-mediated cell lysis and included titrated mixes with normal human serum to gauge complement inhibition functional strength.
Results: EMB (POD13) revealed widespread C3c, C3d, and C4d capillary deposition and endothelial activation, suggesting early innate complement-mediated injury. After initiating eculizumab, upstream complement fragments (C3c, C3d, C4d) remained detectable in biopsies (as expected, given known tissue persistence), but capillary C5b-9 deposition was only focally evident on later biopsy (POD30) (Figure 1). Ex vivo assays indicated that switching from C1INH to eculizumab improved complement control: serum sC5b-9 levels dropped to low levels, and bmHam showed reduced complement-mediated lysis consistent with effective terminal pathway blockade (Figure 2A). In dilutional bmHam tests, patient serum obtained on eculizumab required significantly higher proportions of normal serum to reach 50% cytotoxicity indicating stronger complement blockade compared to serum on C1INH. Notably, plasma exchange (TPE) and massive transfusion events correlated with transient rises in sC5b-9 and bmHam cytotoxicity, suggesting reduced drug levels and complement “breakthrough” that were promptly identified by these assays (Figure 2B).
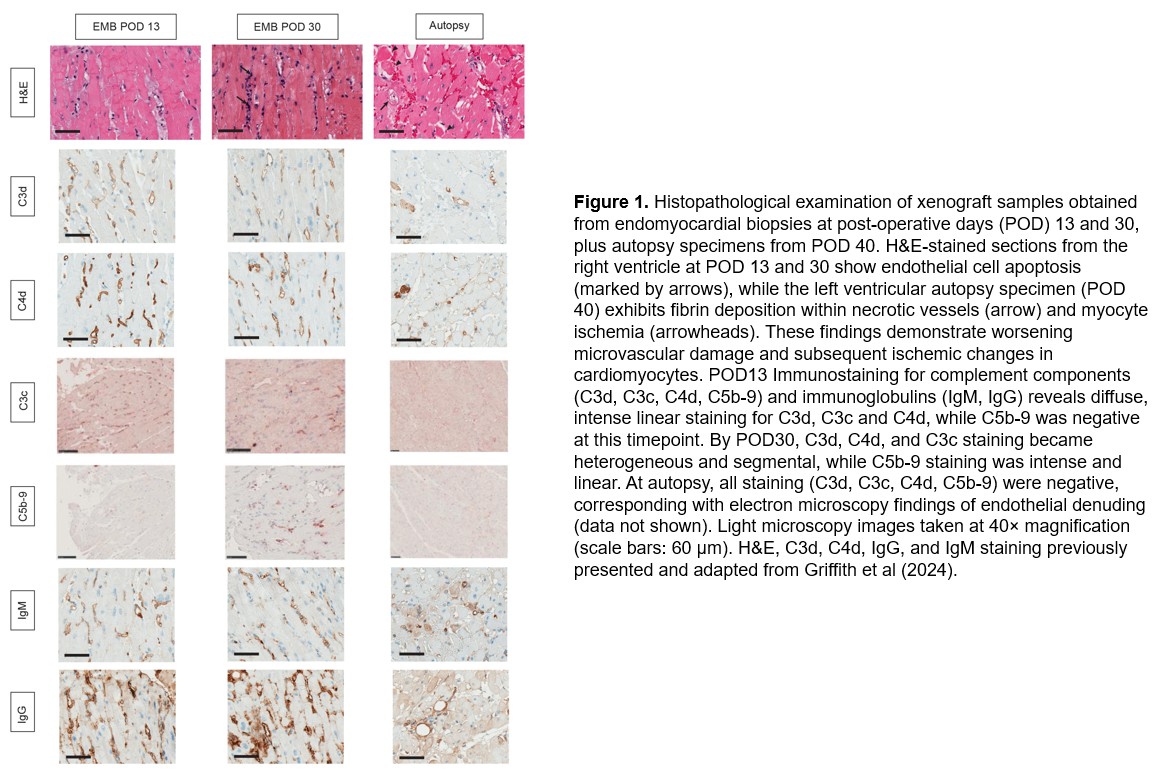
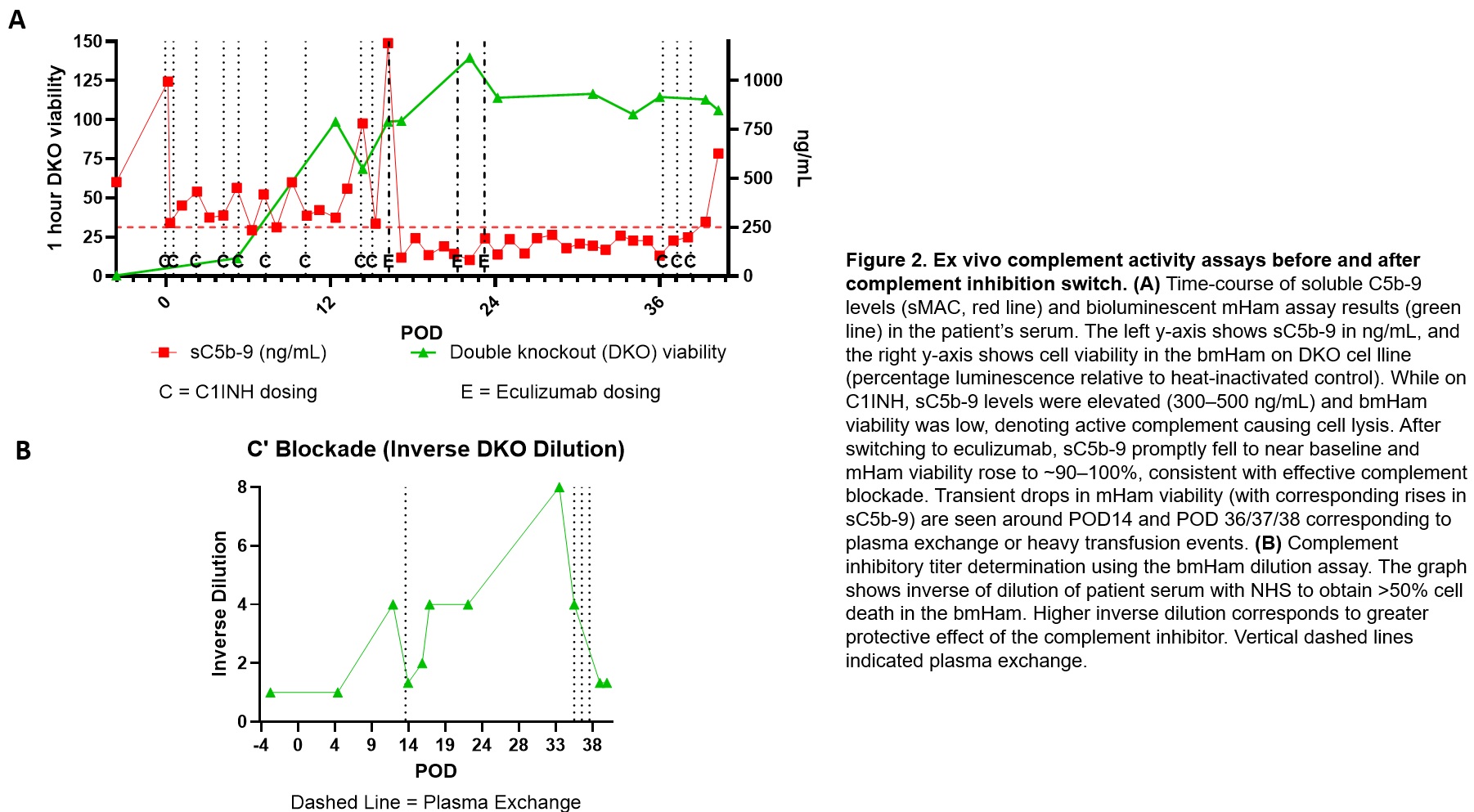
Conclusions: Activation of complement contributed to early porcine-to-human cardiac xenograft injury despite transgenic human complement-regulatory transgene expression. A shift from upstream (C1) to terminal (C5) inhibition aligned with marked reduction in circulating and tissue terminal complement activity. Immunohistochemical detection of C3d/C4d provided evidence of complement activation but could not discriminate ongoing injury due expected persistence of tissue complement deposits. By contrast, real-time assays (sC5b-9 and bmHam) proved valuable for monitoring functional complement activity and guiding therapeutic adjustments.
NIAID/NIH-supported funding that helped generate preliminary data for FDA submission (5U19AI090959). We thank Machaon diagnostics (Mike Ero, Brad Lewis, and Jamey Kain) and their laboratory staff for assistance on measurement of sC5b-9.. We thank L. Faucette and his family for the opportunity to participate in his care, learn alongside his journey and be inspired by his persistence and sense of humor.. We thank the laboratory and clinical staff for their contribution to this case, referred to here as the University of Maryland Cardiac Xenotransplantation Working Group..
[1] Complement
[2] Innate Immunity
[3] Complement Monitoring
[4] Complement Inhibition
[5] Cardiac Xenotransplantation
[6] Xenograft survival