Robert A Montgomery, United States
Professor and Chair of Surgery
Director, NYU Langone Transplant Institute
NYU Langone Health
Longest surviving recipient of a xenotransplantation: Allo-sensitized patient receiving a 10 gene-edited pig kidney
Robert A Montgomery1, Jeffrey Stern1, Adam Griesemer1, Jayme Locke2, Karen Khalil 1, Imad Aljabban1, Tal Eitan1, Ian Jaffe1, Carl Rosenthal1, Elaina Weldon1, Kimberly Sureau1, Nicole Ali1, Sapna Mehta1, David Ayares2, Megan Sykes3, Massimo Mangiola1, Simon Williams1, Brendan Keating1, Ming Wu4, Alexandre Loupy5, Vineeta Kumar6, Edward Skolnik1, Aprajita Mattoo1, Vasishta Tatapudi1.
1NYU Langone Transplant Institute, New York, NY, United States; 2United Therapeutics Corp, Durham, NC, United States; 3Columbia Center for Translational Immunology, Department of Medicine, Columbia University Medical Center, New York, NY, United States; 4Anatomic Pathology, Northwell Health, New Hyde Park, NY, United States; 5Paris Institute for Transplantation and Organ Regeneration, Paris, France; 6Division of Nephrology/Transplant Nephrology, University of Alabama at Birmingham, Birmingham, AL, United States
Introduction: Here we describe the clinical management of the longest living recipient of a porcine xenokidney and the infectious and immunosuppressive complications that eventually led to graft loss.
Methods: A 10 gene-edit porcine kidney (GGTA1, CMAH, B4GALNT2, and GHR gene inactivations; hCD46, hCD47, hCD55, hTHBD, hPROCR, and hHMOX1 human transgene insertions) was transplanted into a 53-year-old female prior living donor, on hemodialysis for 9 years, with a cPRA of 99.98%. No experimental immunosuppressive medications were used.
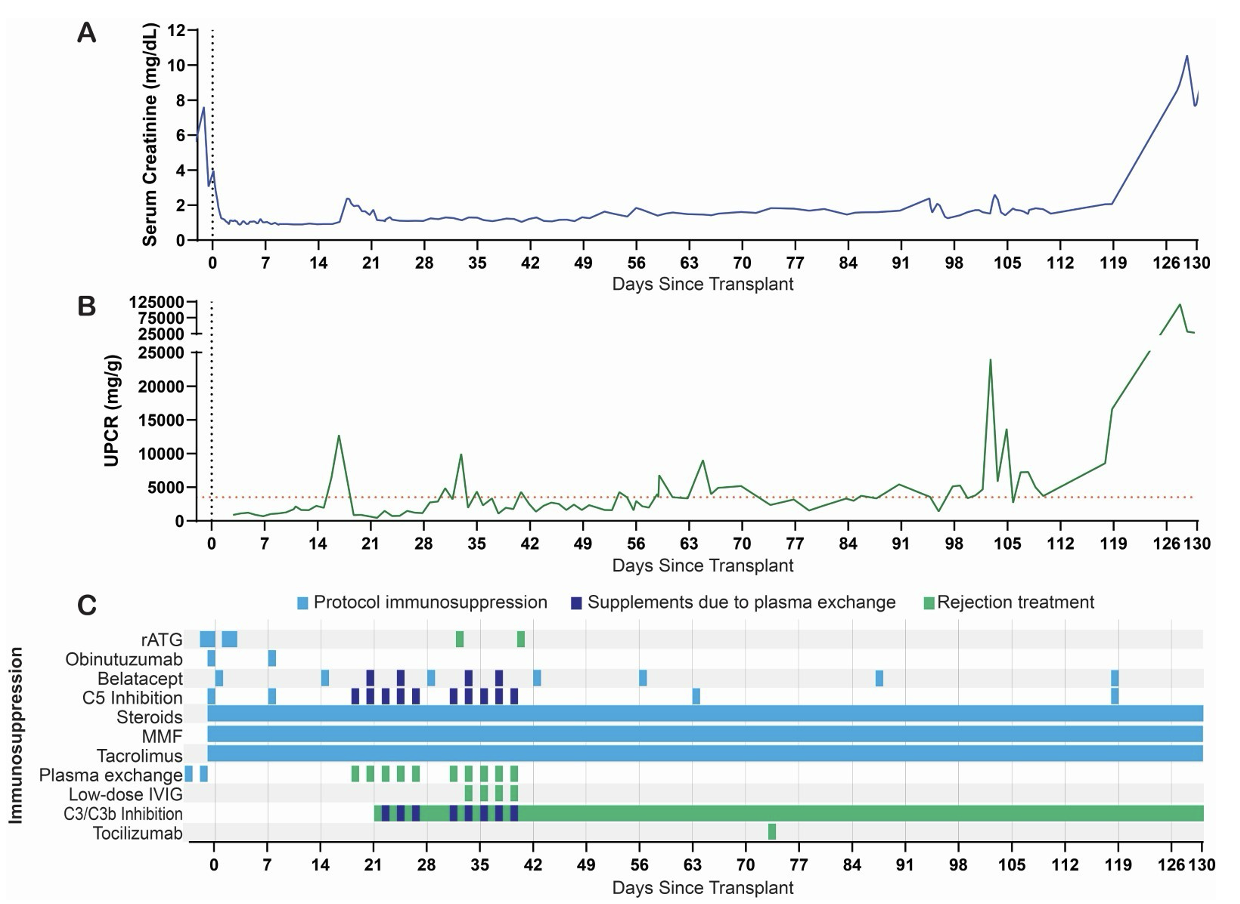
Results: Immediate graft function was evidenced by a mean serum creatine (sCr) and urinary protein-to-creatinine ratios (UPCR) of 1.2 mg/dL and 1.4 g/g, respectively, for the first two weeks post-transplant. UPCR increased to 12.7 g/g along with a rise in IgG and IgM on flow crossmatch. A biopsy performed on POD 17 demonstrated glomerulitis, peritubular capillaritis, diffuse C4d staining (Banff g2 t0 i0 ptc0-1 v0 c4d3, cg0 ct0 mm2 ci0 ti0 cv0 ah0 i-ifta0), C3 deposition in the glomeruli, and 90% podocyte foot process effacement. The AMR treatment plan included 10 plasmapheresis sessions, low dose IVIG, C3/C3b complement inhibitor, and 3 mg/kg rATG. POD 57 and 85 biopsies confirmed absence of AMR and resolution of foot process effacement. On POD 73 the patient was administered IL-6 receptor blockade for a monocyte rich transcriptomics signature on POD 57 biopsy. Following the establishment of histologic immunologic quiescence the patient was started on anti-proteinuric agents to lower her still-present nephrotic range proteinuria. Unfortunately, despite these agents the patient remained with proteinuria that averaged 5.8 ± 5.8 g/g daily. She continued to maintain excellent xenograft function, however, with a stable sCr of 1.59 ± 0.32 mg/dL that increased to 2.06 mg/dL once fully optimized on anti-proteinuric agents. The development of two severe soft tissue infections of the skin, the second of which was accompanied by gram-negative bacteremia prompted the cessation of IL-6 receptor blockade and a reduction in calcineurin inhibitor goal by 50%. The patient was maintained on mycophenolate mofetil, prednisone, belatacept, and complement inhibition. On POD 125, the patient was noted to have a rise in sCr from a baseline of 2.05 mg/dL to 3.99 mg/dL. Her kidney function continued to rapidly decline, with sCr peaking at 10.56 mg/dL. POD 128 biopsy demonstrated loss of normal renal architecture and extensive, severe interstitial hemorrhage. The patient underwent explant on POD 130 and returned to dialysis.
Conclusions: Following successful treatment of an early episode of AMR, we were able to maintain a Xenokidney in a living human being for 130 days, establishing the longest surviving recipient of a xenotransplant. The patient’s xenograft failed in the setting of reduced immunosuppression due to recurrent infections and the likely loss of monoclonal drug in her urine due to persistent, nephrotic range proteinuria.
This data is supported by funding from United Therapeutics Corporation, PBC. Tal Eitan is supported by the National Cancer Institute, National Institutes of Health, through Grant Award Number T32 CA193111.
References:
[1] Xenokidney
Lectures by Robert A Montgomery
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-30 17:15 - 18:45 |
Complement and humoral rejection: Still barriers to success? | DSA and pre-screening | Auditorium |
|
Wed-01 13:30 - 15:00 |
Clinical breaking news / Innovation Supported by:  |
Clinical outcome of pig-to-human kidney xenotransplant in a highly sensitized patient - New York experience | Auditorium |
