Yonsei University
Innate immune–driven fibrotic remodeling as a key driver of chronic kidney xenograft injury
Minsun Jung1, Gaeun Byeon2, Ingyeong Koh2,3, Joon Yong An2,3,4, Il Hee Yun5, Joon Young Jang5, Beom Seok Kim5, Ik Jin Yun6, Jaeseok Yang5.
1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea; 2School of Biosystem and Biomedical Science, College of Health Science, Korea University, Seoul, Korea; 3L-HOPE Program for Community-Based Total Learning Health Systems, Korea University, Seoul, Korea; 4Department of Integrated Biomedical and Life Science, Korea University, Seoul, Korea; 5Department of Internal Medicine, Yonsei University Severance Hospital, Seoul, Korea; 6Department of Surgery, Konkuk University School of Medicine, Seoul, Korea
Introduction: Kidney xenotransplantation is emerging as a promising solution to the organ shortage crisis. However, the molecular mechanisms underlying xenograft failure remain poorly understood. To realize the clinical potential of kidney xenotransplantation, it is critical to elucidate the molecular and cellular characteristics that drive xenograft failure.
Methods: Life-supporting renal xenotransplantations were performed using genetically engineered pigs with targeted coagulation modifications (GE6 to GE8) into cynomolgus monkeys. The immunosuppressive regimen comprised multiple agents, including anti-CD154 monoclonal antibody, abatacept, tocilizumab, crovalimab, corticosteroids, tacrolimus, mycophenolate mofetil, and etanercept. Renal biopsies were obtained at predefined intervals and at euthanasia. Integrative molecular profiling was conducted using bulk RNA sequencing (n=14), single-nucleus RNA sequencing (snRNA-seq, n=15), and spatial transcriptomics (Visium, n=12). Single-cell resolution cell type expression profiles derived from snRNA-seq were mapped onto spatial transcriptomics data using cell2location. Intercellular communication analysis was performed using LIANA and NicheNet to delineate molecular interactions and signaling events.
Results: Histopathological analysis showed that chronic injuries were exclusively identified after week 4 (Table), which was accompanied by a transcriptional transition identified through integrative analysis of bulk and single-nucleus RNA sequencing datasets.
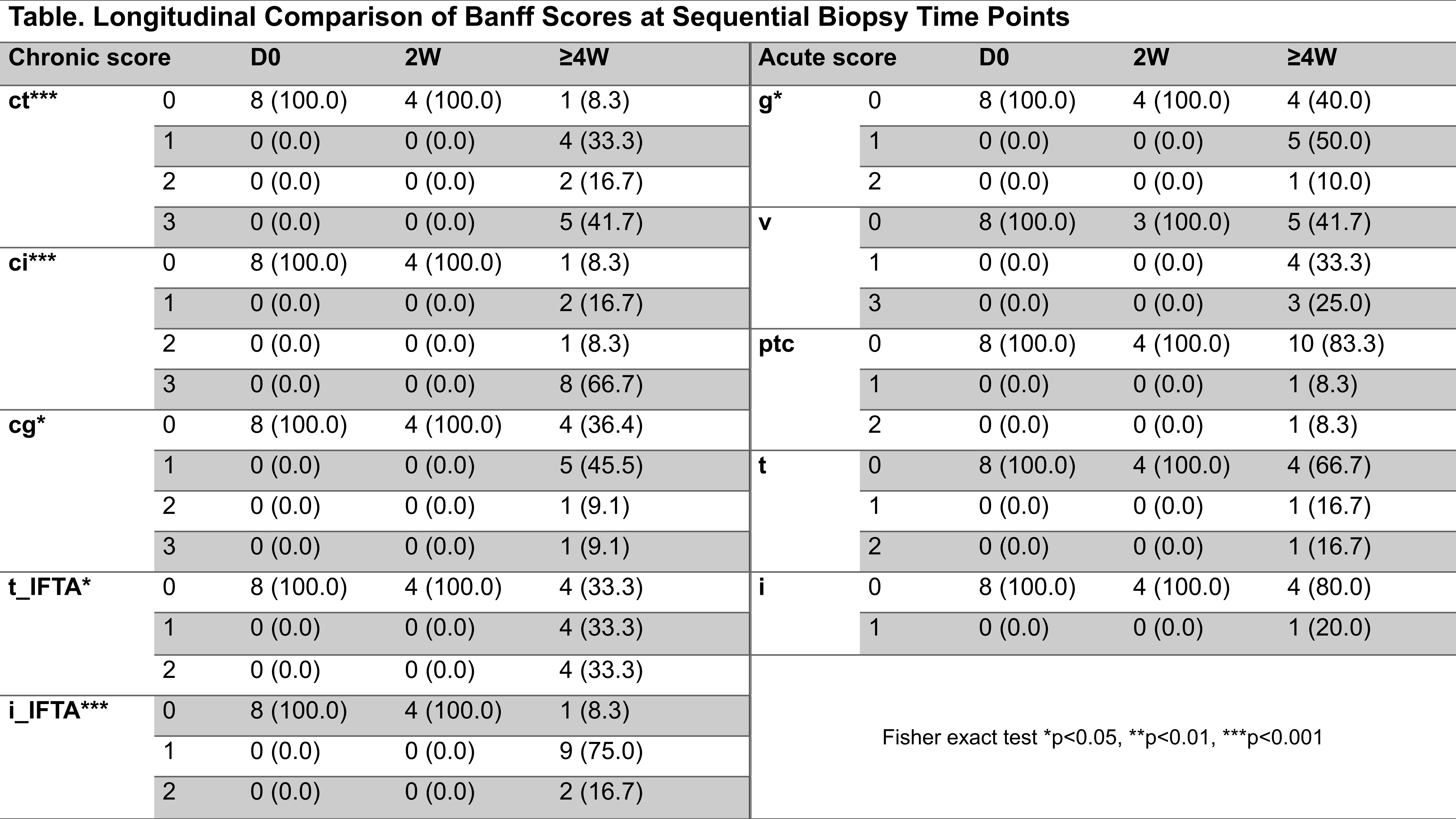
snRNA-seq data identified two noticeable degenerative stromal populations—dFIB and dVSMC/P—marked by high FN1, ACTA2, COL3A1 and COL1A1 expression. Enrichment analysis identified extracellular matrix organization and collagen biosynthesis as the predominant pathways in these stromal populations, reflecting active and pathological extracellular matrix remodeling driven by excessive collagen production and assembly by degenerative stromal cells. Intercellular-communication modeling revealed a directional axis from nonclassical monocytes (ncMonocyte) and M2 macrophages to these stromal cells, with TGF-β1 and SPP1 as principal ligands. Spatial transcriptomics showed TGF-β target genes concentrated around glomeruli (median distance < 500 µm), indicating a periglomerular fibrotic niche driven by innate immune–mediated signaling (Figure).
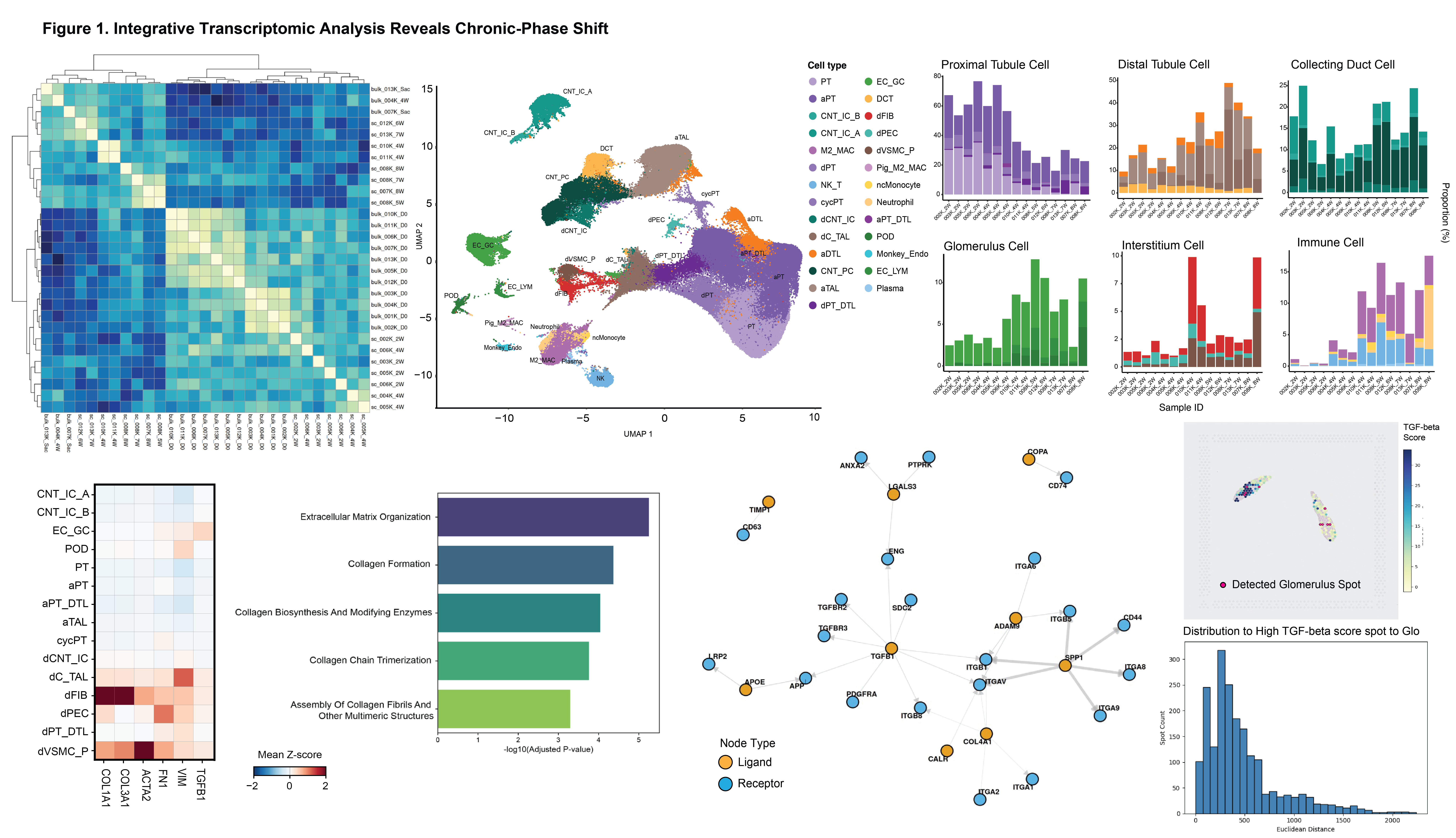
Conclusions: Our findings reveal that innate immune cell–mediated fibrotic remodeling underlies chronic injury in kidney xenografts, with the periglomerular region serving as a focal site of pathological extracellular matrix activation. These results highlight TGF-β1–driven signaling as a pivotal mechanism of chronic graft failure and suggest that targeting innate immune–fibrotic interactions may enhance long-term xenograft survival.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI23C0417). This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (RS-2024-00341570).
References:
[1] Graft Rejection
[2] Xenotransplantation
[3] Gene Expression Profiling
[4] Innate Immunity
[5] Allograft Survival
Lectures by Minsun Jung
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-01 18:45 - 20:00 |
Poster Session 2 - October 1 from 18:45 to 20:00 | Innate immune–driven fibrotic remodeling as a key driver of chronic kidney xenograft injury | Atrium |
