Systemic inflammatory biomarkers versus graft specific hTBM in monitoring xenograft status
Prachi Sardana1, Megan Dufault2, Farzana Rahman1, Amy Dandro1, Kasinath Kuravi 1, Willard Eyestone1, David Ayares1, Richard Pierson 2.
1Transgene Analytics, Revivicor (Subsidiary of United Therapeutics) , Blacksburg, VA, United States; 2Center for Transplantation Sciences and Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Introduction: Xenograft transplantation is a promising treatment for end stage organ failure. However, widespread application remains complicated by biomolecular interspecies incompatibilities, leading to graft rejection. Currently, genetic and therapeutic methods are being developed in pig to non-human primate (NHP) transplant models to prolong graft survival. To monitor graft condition, there is a need to measure several biomarkers in transplant recipients. Systemic inflammatory markers like C-Reactive Protein (CRP), Interleukin-6 (IL-6), and Monocyte Chemoattractant Protein 1 (MCP-1), are well-studied and relatively easy to measure. While CRP/IL-6/MCP-1 provide an understanding of the recipients’ pro-inflammatory status, it may be more useful to study graft specific markers. Humanized thrombomodulin (hTBM) is present in some genetically modified porcine xenografts to prevent pro-coagulatory effects stemming from interactions between porcine TBM and NHP/human thrombin. The following study retrospectively measured CRP/IL-6/MCP-1/hTBM in serum and plasma samples from kidney and heart xenograft baboon recipients, aiming to determine which marker is the best predictor of early graft failure.
Methods: Biological parameters were retrospectively evaluated in plasma and serum samples using ELISA. CRP (cat# CRP-3, Life Diagnostic, Inc., West Chester, PA, USA), IL-6 (cat# BMS641-2, Thermo Fisher, Vienna, Austria), and MCP-1 (cat# EP16RB, Thermo Fisher, Vienna, Austria) were measured on a SpectraMax iD3 ELISA plate reader, following the manufacturers' instructions. Transgenic human-specific TBM (CD141) was measured using an ELISA kit (ab46508, Abcam, Boston, MA) on a Biotek ELISA plate reader, following the manufacturer's instructions.
Results: Kidney recipients presented with an initial spike in CRP, while heart recipients presented with an initial spike in IL-6. Differences in treatment regimen, including the administration of an IL-6 receptor antagonist for heart recipients, may affect the reliance of CRP and IL-6 as inflammatory markers. Kidney and heart recipients presented with similar average MCP-1 trends. While both exhibited stabilized levels following an initial increase within 24 hours of transplant, kidney recipients yielded significantly higher levels of hTBM relative to heart recipients. Unlike CRP/IL-6, hTBM levels remained stable in xenokidney recipients presenting with acute gastric dilatation (AGD) but no early graft failure.
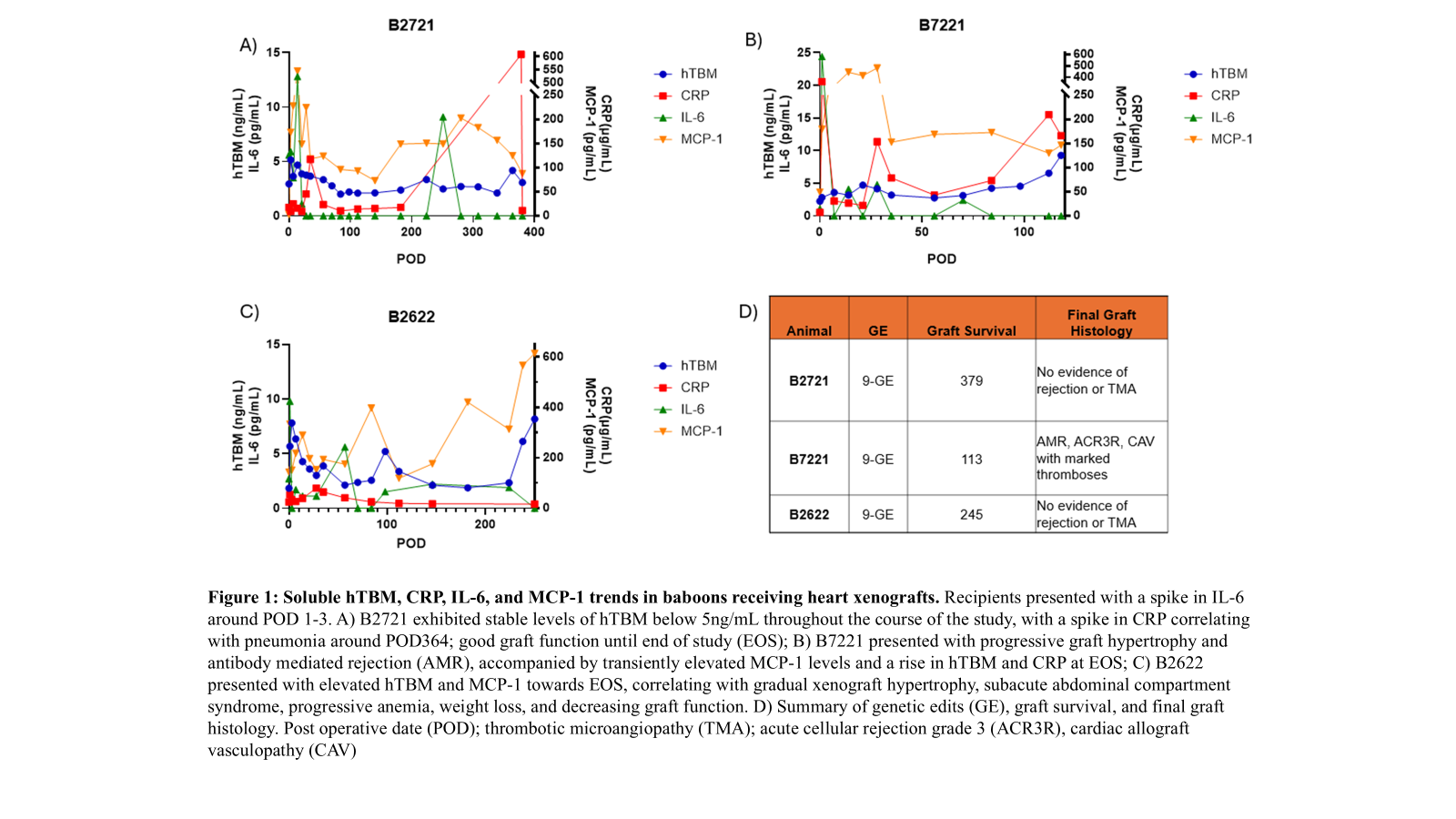
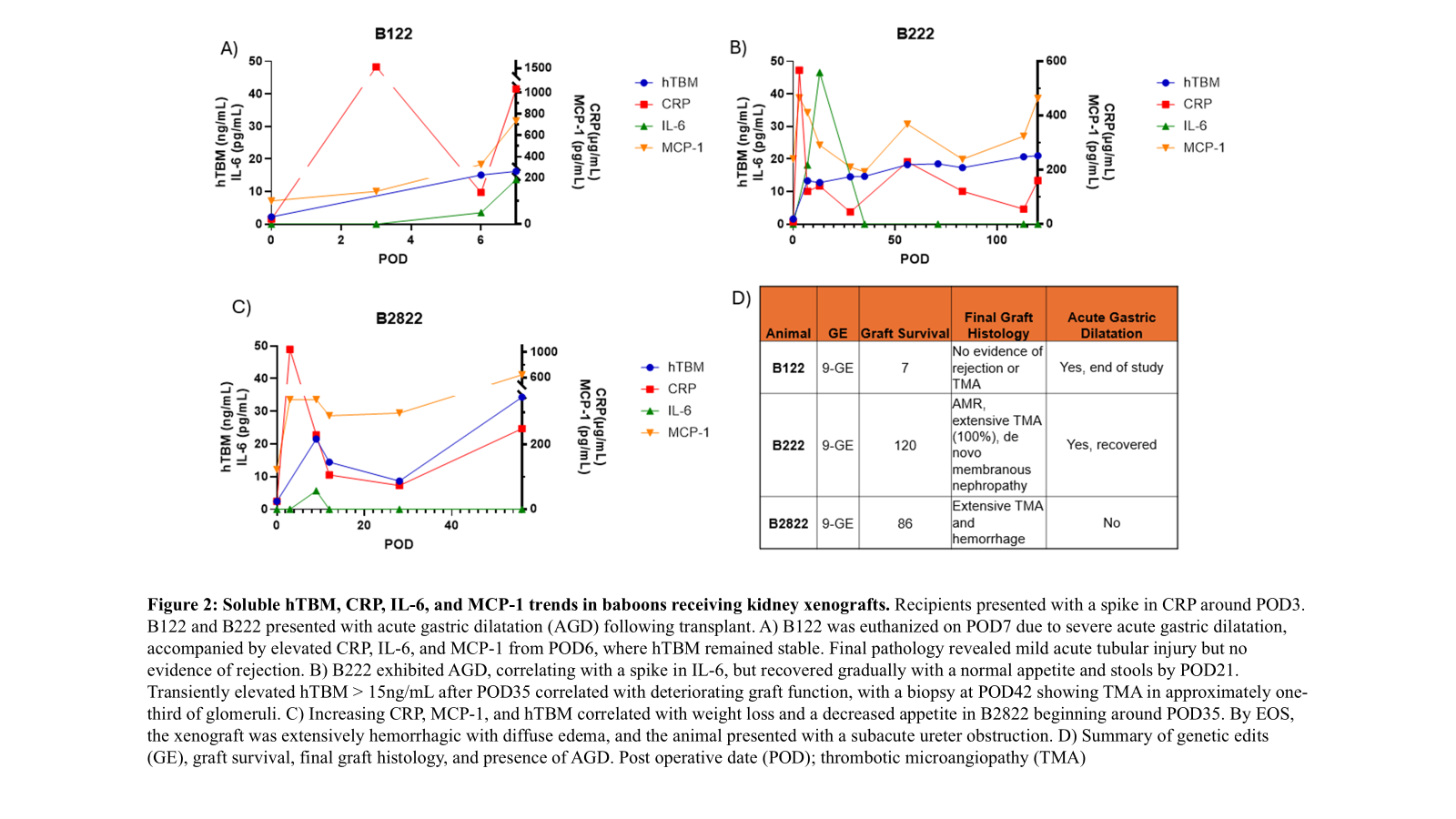
Conclusions: Due to graft-specificity and a significant role in anti-inflammation and anti-coagulation, hTBM may serve as a biomarker to monitor organ status and should be studied further.
Lectures by Farzana Rahman
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-01 18:45 - 20:00 |
Poster Session 2 - October 1 from 18:45 to 20:00 | Systemic inflammatory biomarkers versus graft specific hTBM in monitoring xenograft status | Atrium |
