Ekaterina Umnyakova, Switzerland
Postdoc/assistant
Department of Pharmaceutical Sciences
University of Basel
Exploring the efficacy of complement-modulating protective coatings for porcine endothelial cells via combination of metabolic glycoengineering and click chemistry
Ekaterina Umnyakova1, Jannes Felsch1, Simona Jacquemai1, Shugirshan Suthagar1, Haijie Zhao1, Alexander J. Lander1, Said Rabbani1, Daniel Ricklin1.
1Pharmaceutical Sciences, University of Basel, Basel, Switzerland
Molecular Pharmacy Group.
Introduction: During xenotransplantation, activation of host defense mechanisms, particularly the complement cascade, can induce inflammatory responses that result in xenograft dysfunction or rejection. Protective surface coatings capable of modulating complement activation confer a promising therapeutic approach to enhance xenograft viability, yet their evaluation demands specialized experimental models that enable biocompatible cell modification and mimic xenotransplant conditions.
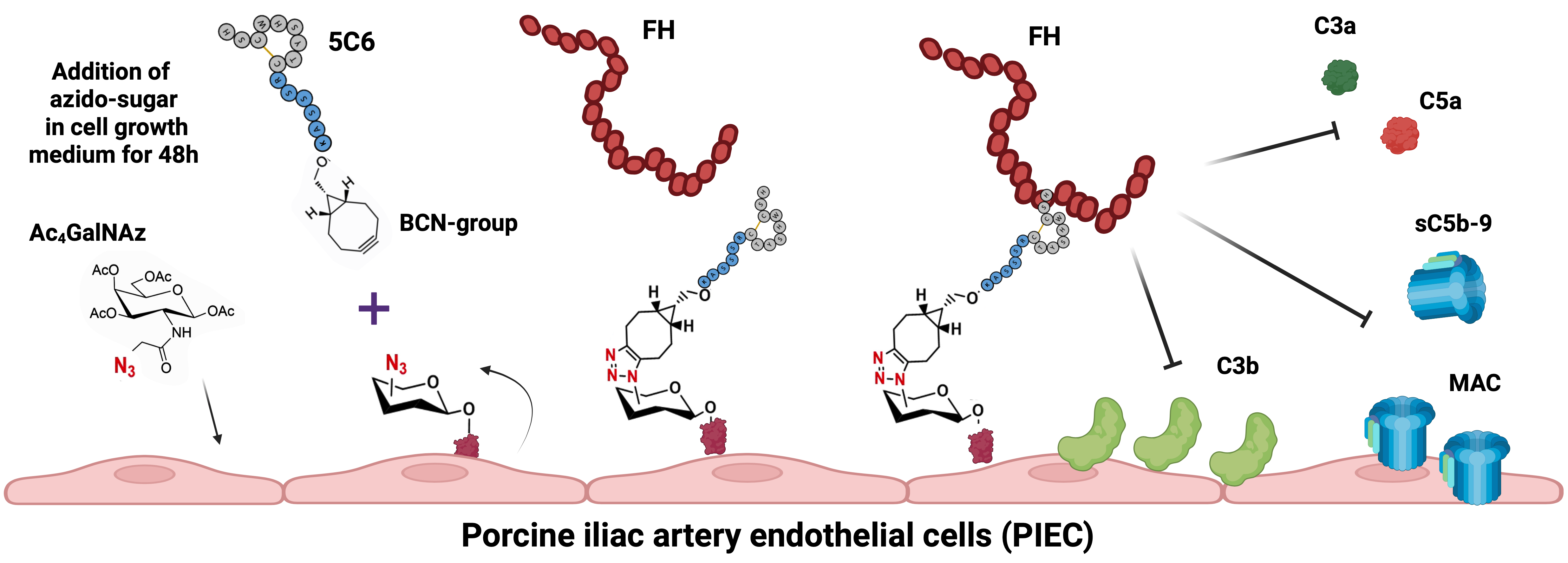
Methods: We developed a coating approach for endothelial cell models by integrating metabolic glycoengineering with bioorthogonal click chemistry. Porcine iliac artery endothelial cells (PIECs), relevant to xenotransplantation applications, were cultured in the presence of azide-modified monosaccharides to introduce reactive azide groups onto cell-surface glycans. This enabled the covalent attachment of an alkyne-functionalized peptide (5C6) that specifically recruits the physiological complement regulator factor H (FH; Fig.1A). The surface distribution of azides, coated peptides, and recruited FH was monitored by fluorescence microscopy and functional efficacy by measuring opsonization upon serum exposure.
A
B
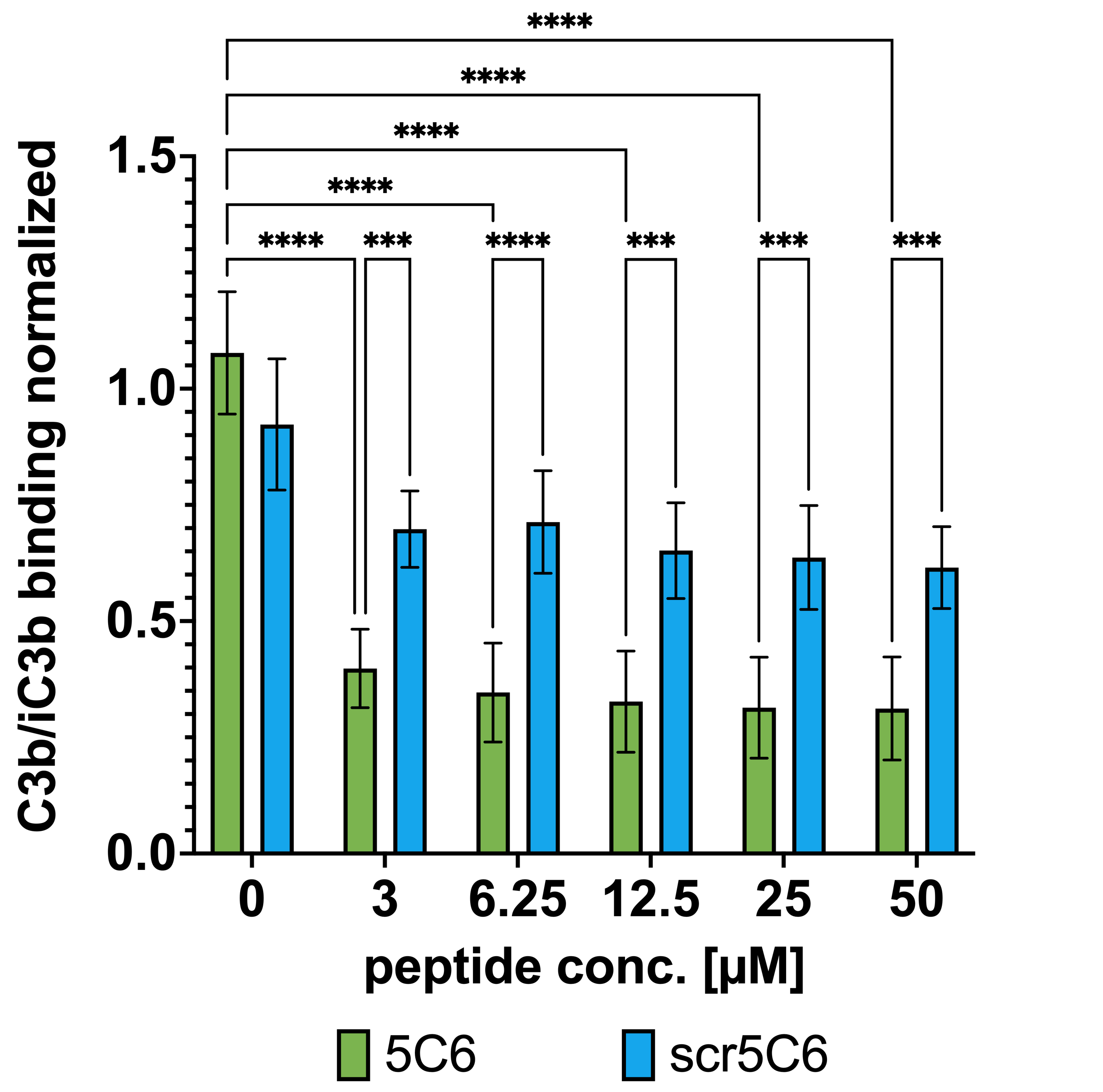
Figure 1. (A) Coating approach to provide complement control via regulator recruitment. Incubation of endothelial cells in Ac4GalNAz-supplemented culture medium leads to the incorporation and presentation of azido-groups on the glycocalyx. This allows the covalent coupling of a clickable 5C6 derivative to azide-bearing glycans via a triazole bridge. 5C6-coating of cell surfaces enables the recruitment of endogenous FH, downregulating opsonization with C3b, MAC formation, and the release of anaphylatoxins (C3a, C5a). (B) Complement downregulation was evaluated in 50% Mg2EGTA serum using different concentrations of peptides 5C6 and scr5C6 (scrambled negative control). Bars indicate the mean of the replicates; error bars indicate standard deviation, ****: significant (p ≤ 0.0001).
Results: By optimizing conditions for glycan modification, we achieved peptide coating of PIEC with controllabledensity and even surface distribution while preserving endothelial cell viability. PIEC coated with 5C6, but not with a control peptide (scr5C6), efficiently recruited FH from purified sources and normal human serum onto 5C6. When exposed to Mg2EGTA serum, 5C6-coated cells showed enhanced resistance to alternative pathway-mediated complement attack (Fig.1B). Beyond efficacy screening, this study provided critical insights into mechanisms underlying glycan modification, peptide coating stability, and complement activation dynamics specific to endothelial cells in xenotransplantation.
Conclusion: Our endothelial cell-based system offers a powerful platform for screening and optimizing complement-regulating coatings for xenotransplantation and may facilitate broader mechanistic studies and preclinical evaluation in xenotransplantation settings.
References:
[1] Metabolic glycoengineering
[2] Click chemistry
[3] porcine endothelial cells
[4] functionalized coatings
[5] complement system
[6] factor H
[7] factor H binding peptides
[8] complement modulation
Lectures by Ekaterina Umnyakova
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-02 15:05 - 15:55 |
Innate Immunity and Inflammation 1 | Exploring the efficacy of complement-modulating protective coatings for porcine endothelial cells via combination of metabolic glycoengineering and click chemistry | H8-01-F |
