Research Fellow
Department of Surgery, Center for Transplantation Sciences
Massachusetts General Hospital
Combined 11-gene-editing with HLA-E expression and donor macrophage depletion extends survival in pig-to-baboon lung xenotransplantation
Sho Takemoto1, Seyed Amir Sanatkar1, Zahra Habibabady1, Sara De Taeye1, Ike Ileka1, Kohei Kinoshita1, Victoria Diaz1, Megan Dufault1, Lars Burdorf2, Will Eyestone2, David Ayares2, Richard N Pierson III1.
1Department of Surgery, Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States; 2Revivicor Inc., Blacksburg, VA, United States
Introduction: Lung xenotransplantation offers a potential solution to the critical shortage of donor lungs, yet significant immunological and physiological barriers remain. Key challenges include maintaining vascular barrier function, preventing coagulopathy, and mitigating innate immune responses, particularly those mediated by macrophages and NK cells. The longest reported survival in pig-to-baboon lung xenotransplant is 31 days, achieved with a double knockout (α1,3-GalT, β4GalNT2) based 7GE pig. We hypothesized that a more comprehensive multi-gene-editing, targeting NK cell activation and donor macrophage depletion, could prolong graft survival. This study aimed to assess the function and survival of 10 and 11GE pig lungs in parallel in vivo (pig-to-baboon) and ex vivo (pig-to-human) xenotransplant models.
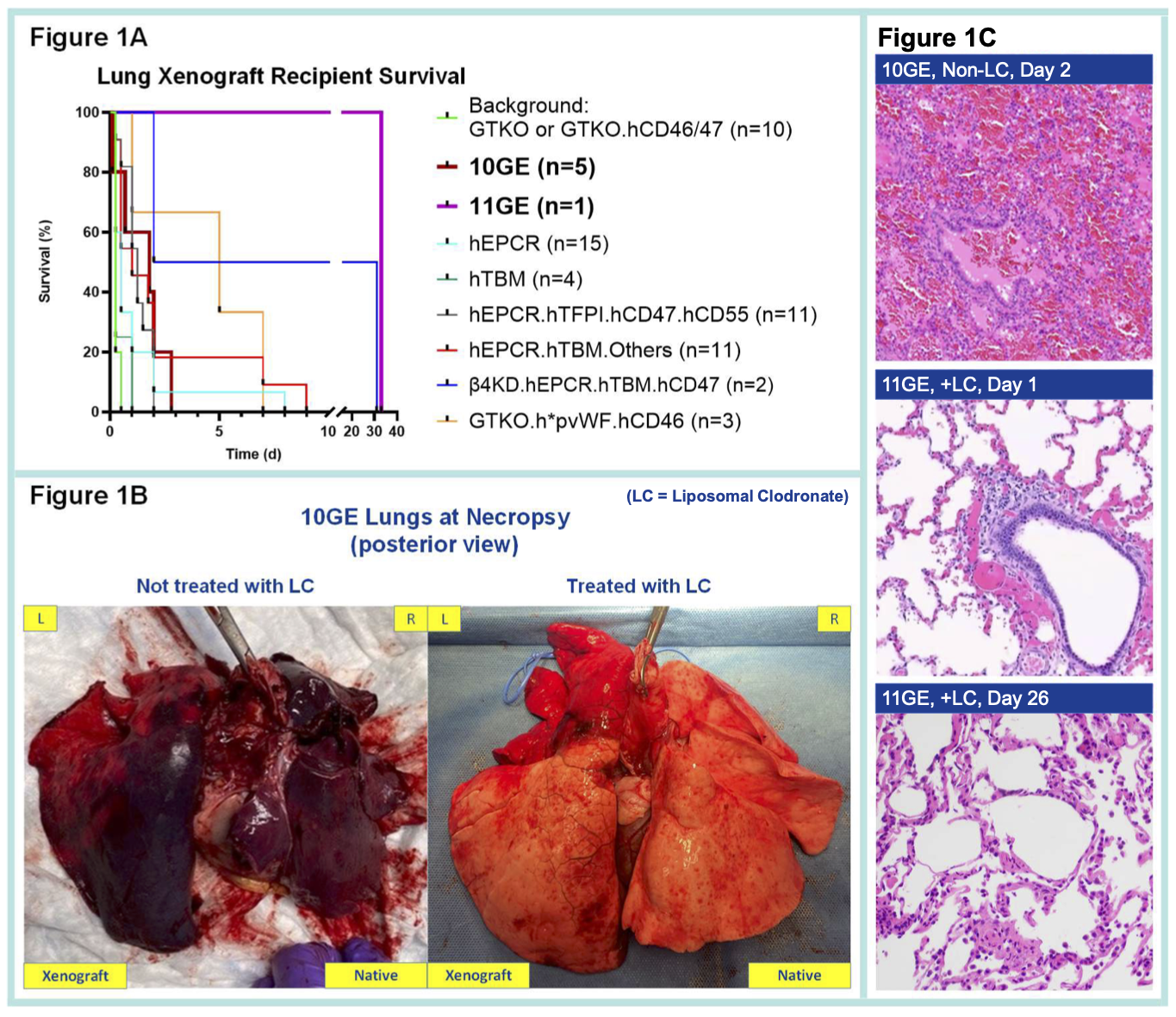
Methods: Left single lung transplants were performed in baboons using lungs from 10 (n=5) and 11GE (n=1) pigs. The 10GE pigs had growth hormone receptor and triple carbohydrate gene knockout (TKO) (α1,3-GalT, β4GalNT2, and CMAH), and 6 added transgenes (hCD46.hCD55.hEPCR.hTBM.hHO-1.hCD47); the 11GE pig additionally expressed human HLA-E. Donor pigs were pre-treated with desmopressin to deplete porcine vWF (pvWF), with three receiving liposomal clodronate (LC) for macrophage depletion. Recipient treatment included splenectomy, αCD20, ATG, CoVF, donor kidney perfusion (3 of 6), selectin/integrin blockers, αGP1b, 1-BIA, α1AT, αCD154, and inhibitors of C1 esterase, IL-6, IL-8, and TNFα. The contralateral right lungs were perfused ex vivo with fresh human blood treated with αGP1b, 1-BIA, and selectin blockade.
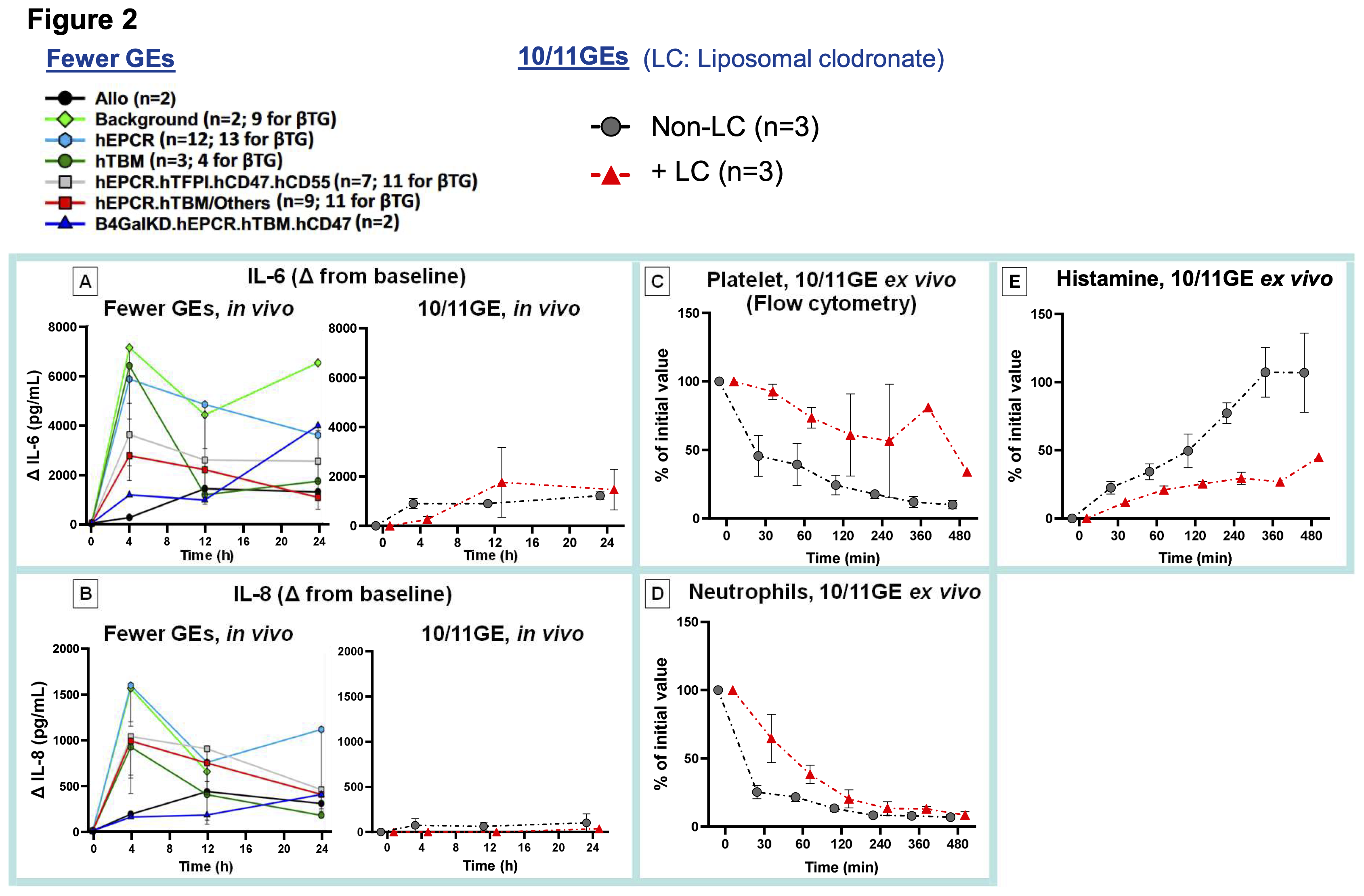
Results: The 11GE lung survived 33 days, and 10GE lung survivals were 1 to 3 days in vivo (Figure 1A) and >8 hours ex vivo. 10GE lung failure in vivo was primarily due to loss of vascular barrier function despite markedly reduced levels of IL-6 and IL-8 compared to reference GE lungs (Figure 2A-B). LC-lungs had less severe parenchymal damage in vivo (Figure 1B). Histopathology confirmed extensive inflammation, diffuse hemorrhage, and microthrombosis in non-LC 10GE lungs; in contrast, the 11GE+LC lung showed only mild inflammation at POD 1 and preserved alveolar structure and airway epithelium at POD 26 (Figure 1C). Ex vivo, neutrophil and platelet sequestration were delayed, and histamine levels were reduced relative to non-LC lungs (Figure 2C-E).
Conclusion: The 11GE lung, combined with donor macrophage depletion, resulted in a record 33-day survival, surpassing the previous 31-day record achieved with a 7GE lung lacking CMAH-KO and the 7-day survival of humanized pvWF (h*pvWF) expressed lung. Donor macrophage depletion attenuates xenograft injury, in part by impacting neutrophils and platelets, and reducing histamine release. Some combination of omitting the CMAH-KO (9GE), additional expression of h*pvWF and/or HLA-E, and donor macrophage depletion may synergistically extend lung xenograft survival in baboons.
References:
[1] Xenotransplantation
[2] Xenograft Survival
[3] Gene-edited Pig
[4] Lung Xenotransplantation
[5] Pig-to-Baboon Transplant Model
[6] Preclinical Study
[7] Ex Vivo Perfusion Model
Lectures by Sho Takemoto
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-02 15:05 - 15:55 |
Pre- and Subclinical Models 1 | Combined 11-gene-editing with HLA-E expression and donor macrophage depletion extends survival in pig-to-baboon lung xenotransplantation | Auditorium |
|
Thu-02 16:20 - 17:05 |
Small Animals and In Vitro | Modulating human neutrophil rolling on porcine endothelium: Effects of Sialoadhesin and GPIb blockade combined with genetic engineering under microvascular flow | H8-01-D |
