Attenuation of platelet activation and thrombosis in a dynamic shear flow model with GalTKO.hCD55.hTBM porcine endothelium
Megan Dufault1, Seyedamir Sanatkar1, Zahra Habibabady1, Victoria Diaz1, Jonathan Schulz1, Caroline Gomes Lucas2, Kevin D Wells2, Kristin M Whitworth2, Richard N Pierson III1.
1Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States; 2National Swine Resource and Research Center (NSRRC), Columbia, MO, United States
Introduction: Long term xenograft survival is hindered by coagulation pathway dysregulation, marked by the inefficient binding of inactivated protein C (PC) to porcine thrombomodulin (TBM). The TBM/thrombin-mediated cleavage of PC into an active form drives anti-coagulatory, anti-inflammatory, and anti-apoptotic pathways through the endothelial protein C receptor (EPCR). This study evaluates the effect of hTBM inclusion in the porcine genome on platelet activation, adhesion, and aggregation within a dynamic in vitro xenoperfusion model.
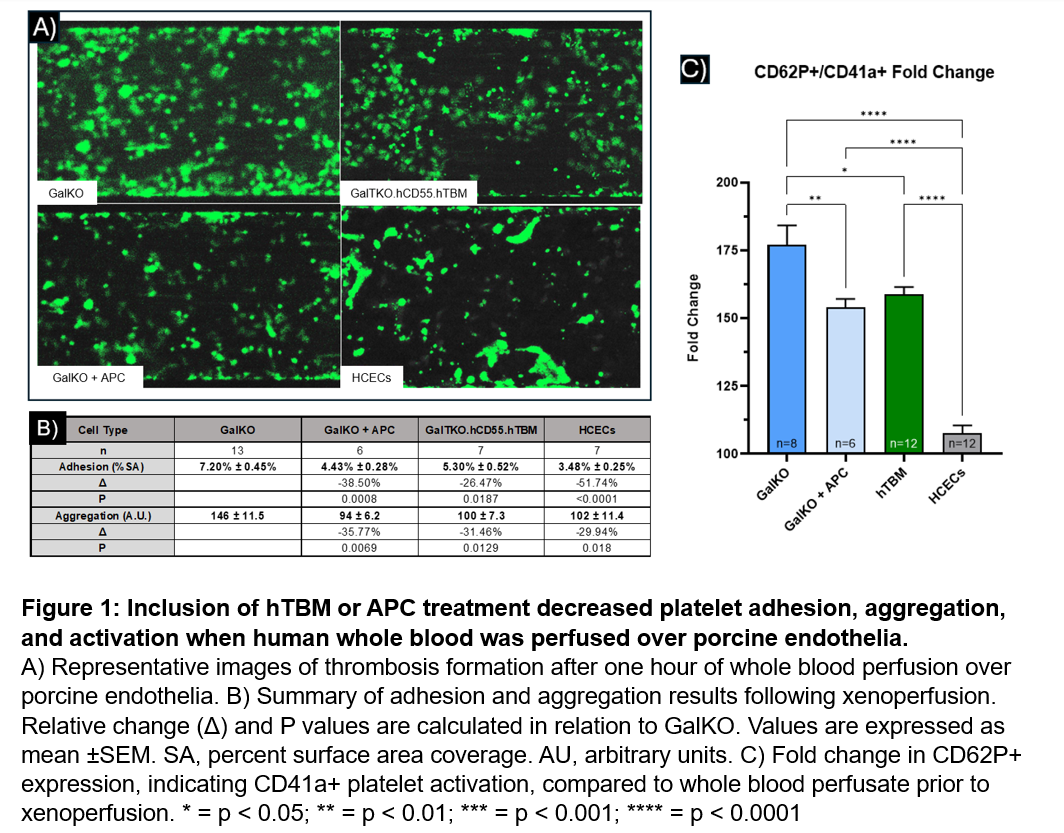
Methods: Genetically modified porcine aortic endothelial cells (PAECs) from GalKO (n=13) and GalKO. β4GALKO.NT2LKO.hCD55.hTBM (n=7) pigs (GalTKO.hCD55.hTBM) between passages three to six and immortalized human coronary endothelial cells (HCECs, n=7) were cultured to confluence in microfluidic channels then treated for 2.5hours with 20ng/mL rhTNF-α. One additional group of GalKO cells were treated with 5.5µg/mL human APC to mimic the intended functionality of hTBM (n=6). Channels were perfused at 5 dynes/cm2 at 37°C for one hour with human whole blood stained with 0.5mM Calcein to visualize thrombosis formation with sequential images generated via Bioflux Montage software (Cell Microsystems) over the duration of perfusion. Blood was collected via slow drip into a sodium heparin tube to minimize platelet activation prior to perfusion. Before and after perfusion, perfusate was collected and fixed in 1% PFA for CD62P analysis via flow cytometry (GalKO n=8; GalKO+APC n=6; GalTKO.hCD55.hTBM n=12; HCECs n=12). CD62P+ platelets were analyzed as frequency of the CD41a+ parent population.
Results: Platelet adhesion after one hour, measured as percent of surface area coverage, was significantly reduced with GalKO+APC (SA 4.43%±0.028%, p=0.0008), GalTKO.hCD55.hTBM (SA 5.30%±0.052%, p=0.0187), and HCECs (SA 3.48%±0.25%, p<0.001) relative to GalKO (7.20%±0.45%). Platelet aggregation, measured as total fluorescence, was significantly reduced with GalKO+APC (94±6.2, p=0.008), GalTKO.hCD55.hTBM (100±7.3, p=0.015), and HCECs (102±11.4, p=0.018) relative to GalKO (146±11.5) (Figure 1B). Fold change in frequency of circulating CD62P+ platelets by flow cytometry before and after xenoperfusion was significantly decreased in GalKO+APC (154.1±3.1, p=0.0097), GalTKO.hCD55.hTBM (158.8±2.7, p=0.0164), and HCECs (107.6±2.9, p<0.0001) relative to GalKO (176.9±7.3). CD62P+ frequency was significantly lower in HCECs than GalKO+APC and GalTKO.hCD55.hTBM PAECs (p<0.0001) (Figure 1C).
Conclusion: Conditions mimicking microcirculatory flow reveal an anti-thrombotic role of hTBM when PAECs are perfused with human blood. The inclusion of hTBM in the porcine genome is a promising method to combat dysregulated platelet driven thrombosis in in vivo xenotransplant models.
References:
[1] Coagulation
[2] Thrombosis
[3] Thrombomodulin
[4] Xenoperfusion
