Establishment of a designated pathogen-free (DPF) donor pig population of genetically edited pigs in Neijiang, China
Xing Xiangyang1, Yang Hengyu1, Gong Manlin1, Pan Dengke1, Deng Shaoping1.
1Chengdu Clonorgan Biotechnology Co., Ltd, Chengdu, People's Republic of China
Introduction: As xenotransplantation progresses toward clinical translation, cross-species transmission of porcine-derived pathogens, particularly porcine cytomegalovirus (PCMV) and porcine lymphotropic herpesviruses (PLHV), represents a critical biosafety challenge in clinical use. Based on our newly-established designated pathogen-free (DPF) pig facility in Neijiang, China, this initiative aims to establish and develop China’s first self-sustaining DPF Bama miniature pig population as a biosafe donor source for clinical xenotransplantation.
Methods: From our Phase I conventional breeding cohort (n=425), 64 representative individuals (15% stratified sampling) underwent comprehensive microbial screening for xenotransplantation-relevant pathogens (PCMV, PLHV-1/2/3), and vertically transmitted pathogens.
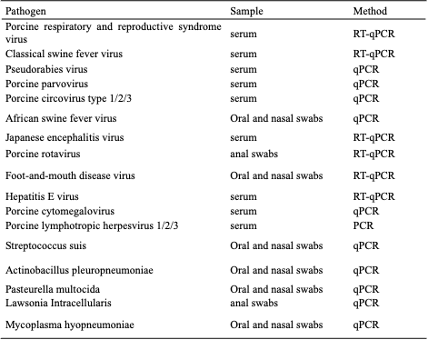
Selected pathogen-negative sows underwent aseptic cesarean section in a DPF barrier facility. Derived piglets received sterilized colostrum to establish the foundation (F₀) generation under strict bio-surveillance, including HEPA -filtered air handling, irradiated feed, and filtered water systems. Longitudinal pathogen monitoring encompassed biweekly blood, fecal, and nasal swab analyses during barrier maintenance.
Results: Initial screening revealed complete absence of vertical transmission pathogens (pseudorabies, parvovirus, circovirus, PRRSV), enabling successful DPF derivation. Xenozoonotic risk profiling identified PCMV (6%), PLHV-2 (22%), PLHV-3 (50%), with notable absence of PERV-C. Through a 13-month surveillance period, 42 F₀ DPF pigs across 8 production batches demonstrated sustained pathogen-free status (90% hysterectomy survival rate), achieving complete elimination of target pathogens (PCMV, PLHV-2/3, parvovirus, Actinobacillus, Streptococcus). The breeding area currently maintains 15 genetically modified (6-gene edited) founders aged 6-12 months, with F₁ progeny successfully generated. In clinical studies including renal xenotransplantation trial demonstrated the living patient survival exceeding 50 days with maintained pathogen-negative status (unpublished data).
Conclusion:This study establishes the China's first DPF Bama miniature pig population for xenotransplantation through integrated pathogen eradication and advanced biocontainment strategies. Our multilayered approach combining hysterectomy derivation, sterile nutritional protocols, and pathogen surveillance demonstrates the feasibility of generating biosafe donors for clinical xenotransplantation, being able to address critical safety barriers in cross-species transplantation and advance the clinical application of xenotransplantation technology.
References:
[1] Xenotransplantation
[2] Designated pathogen-free pig population
[3] Biosafety
[4] Bama miniature pig
Lectures by Xing Xiangyang
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-30 16:20 - 17:10 |
Methodology / Technical Resources | Establishment of a designated pathogen-free (DPF) donor pig population of genetically edited pigs in Neijiang, China | H8-01-F |
