Differential pig and human protein abundance in plasma of patients exposed to pig hepatocytes following bioartificial liver treatments
Angeles Baquerizo1, Vadim Jucaud1, Abdul Rahim Chethikkattuveli Salih1, Ali Keshavarz1, James Markmann2.
1Terasaki Institute of Biomedical Innovation , Los Angeles , CA, United States; 2Transplant Surgery, Hospital of the University of Pennsylvania, Philadelphia, PA, United States
Introduction: Recent advances in pig-to-human xenotransplantation using genetically modified pigs underline the significant clinical potential. Bioartificial liver (BAL) therapy containing wild-type (wt) pig hepatocytes offers a unique clinical model to investigate the xenogeneic immune response in humans. Understanding the dynamics of human and pig protein abundance following BAL treatments is critical for optimizing future pig-to-human xenotransplant strategies.
Objective: Characterize pig and human-specific protein abundance in plasma of patients exposed to pig hepatocytes during BAL treatment, using nanoparticle-based enrichment technology and quantitative mass spectrometry.
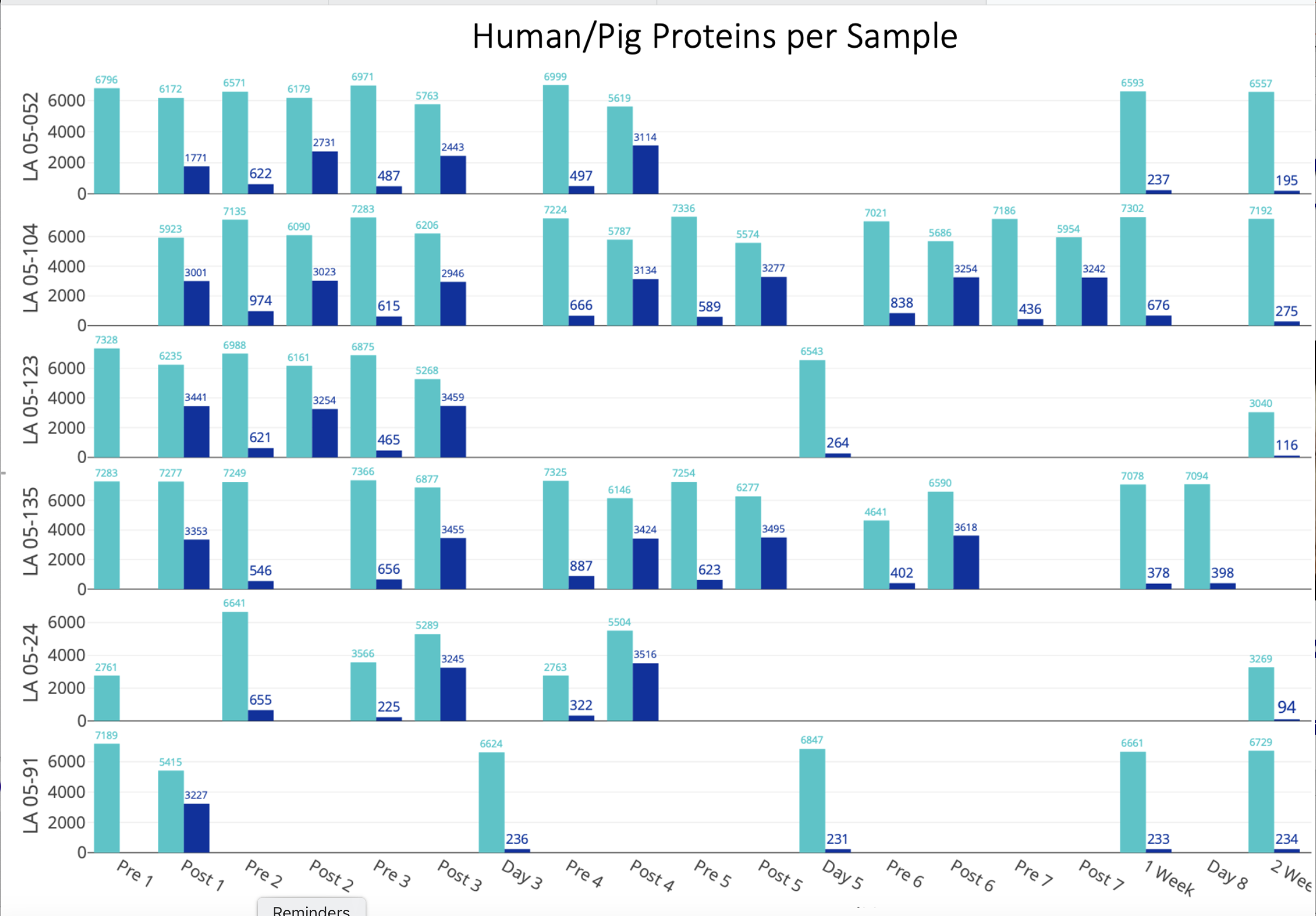
Methods: Six patients who received 1 to 7 BAL treatments were included. Each BAL treatment consisted of six hours of plasma perfusion through a cartridge containing wt pig hepatocytes. Patients receiving multiple treatments, the BAL was repeated within 24 hours. Plasma samples were collected pre-treatment (“Pre”) and serially for two weeks after the BAL treatments. Additionally, plasma samples were obtained from the BAL tubing as plasma returned to the patients after completion of the BAL treatment (“Post”). Controls included normal human and wt pig plasma. A total of 80 plasma samples underwent high-throughput proteomic analysis using the Seer Proteograph™ Assay (nanoparticle-based enrichment) and quantitative mass spectrometry to identify and quantify human and pig plasma proteins across all time points.
Results: Quantitative mass spectrometry analysis (DIA-NN v1.8.1) using human and pig UniProt databases, identified 159,601 unique peptides at 1% FDR, including 65,093 human-specific and 25,539 pig-specific peptides. These species-specific peptides were reconstructed into 8,744 human and 4,225 pig protein groups. The graphic shows temporal trends in human (light blue) and pig (dark blue) plasma proteins at different time points. Baseline analysis of the human protein before pig hepatocyte exposure showed inter-individual variability in human protein abundance, indicative of heterogeneous pre-existing immune status. Immediately following BAL treatment (“Post”), an average of 3,157±415 pig proteins were detected in the plasma returning to the patient, confirming the significant transfer of pig proteins into patient circulation during BAL treatments. Within 24 hours after the last BAL and before the subsequent BAL treatment (“Pre”), pig protein levels markedly decreased to an average of 581±175, suggesting rapid clearance and/or immune-mediated degradation of the xenogeneic proteins.
Conclusions: This study provides the first characterization of human plasma proteome following exposure to pig hepatocytes during BAL therapy. Pig proteins were transferred to patients during BAL treatment, followed by rapid pig protein decline within 24 hours, suggesting efficient clearance and early immune processing, key insights for advancing pig-to-human xenotransplantation strategies.
References:
[1] Xenotransplantation
[2] Bioartificial Liver
[3] Plasma Proteome
[4] Xenoimmune response
Lectures by Angeles Baquerizo
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-30 16:20 - 17:10 |
Organ Xenotransplant - Clinical Application 2 | Differential pig and human protein abundance in plasma of patients exposed to pig hepatocytes following bioartificial liver treatments | Auditorium |
